Anticancer active molecular skeleton 1,4-enyne compound, and preparation method and application thereof
A technology of molecular skeleton and anti-cancer activity, applied in the field of organic chemical synthesis, can solve the problems of cumbersome post-processing, limited substrate scope, less success, etc., to achieve good anti-cancer activity, wide application range, and cell growth inhibition effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A, under room temperature conditions, benzaldehyde (10mmol), methyl acrylate (10mmol) and DABCO (15mmol) are added in the tetrahydrofuran solution successively, stir a week, obtain reaction mixture; Reaction equation is:
[0030]
[0031] B. The solvent was removed from the reaction mixture under reduced pressure, and the residue was purified by silica gel chromatography with petroleum ether and ethyl acetate (PE / EA) to obtain 2-(hydroxyphenylmethyl)-methyl acrylate as a white solid.
Embodiment 2
[0033] (1) In a 10mL Shrek tube, under argon atmosphere, add 2-(hydroxyphenylmethyl)-methyl acrylate (0.5mmol), triisopropylsilylacetylene (0.2mmol), tetrakis (tri Phenylphosphine)palladium (1mol%), calcium bis(trifluoromethylsulfonyl)imide (5mol%) and ammonium hexafluorophosphate (5mol%) were added to N,N-dimethylacetamide (2mL), Stir the reaction at 100°C, the reaction equation is:
[0034]
[0035] (2) After TLC monitors that the reaction is complete, the reaction solution is extracted, and the solvent is removed with a vacuum rotary evaporator, and the product is separated by thin-layer chromatography. The developer is a sherwood oil / ethyl acetate system, and the product is light yellow oil 2- Benzyl-5-(triisopropylsilyl)pent-4-ynoic acid methyl ester, yield 91%.
Embodiment 3
[0037] (1) In a 10mL Shrek tube, under argon atmosphere, add cinnamyl alcohol (0.2mmol), 4-ethynyl anisole (0.5mmol), tetrakis(triphenylphosphine) palladium (1mol%), bis Calcium (trifluoromethylsulfonyl)imide (5mol%) and tris(dimethylamino)phosphorus (1.2mol%) were added to N,N-dimethylacetamide (2mL), and stirred at 100°C for reaction, The reaction equation is:
[0038]
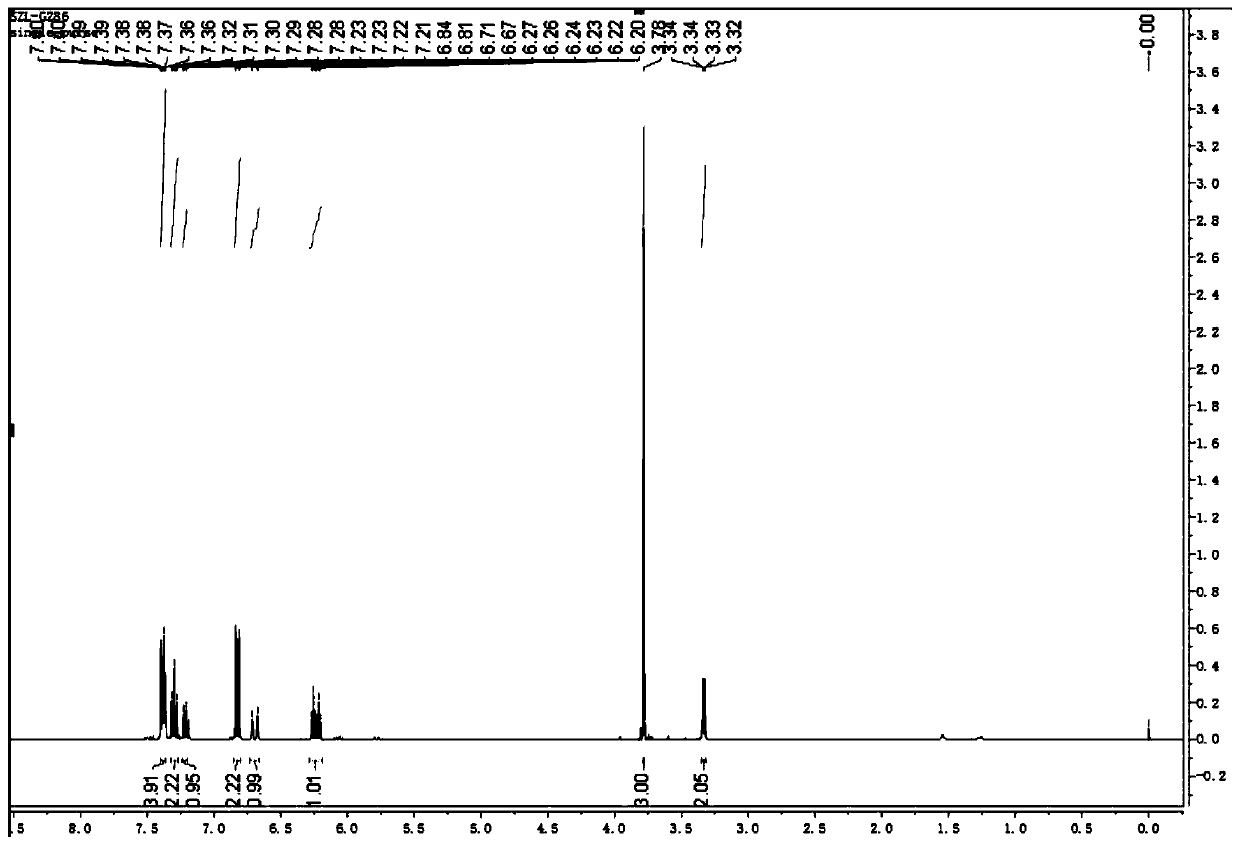
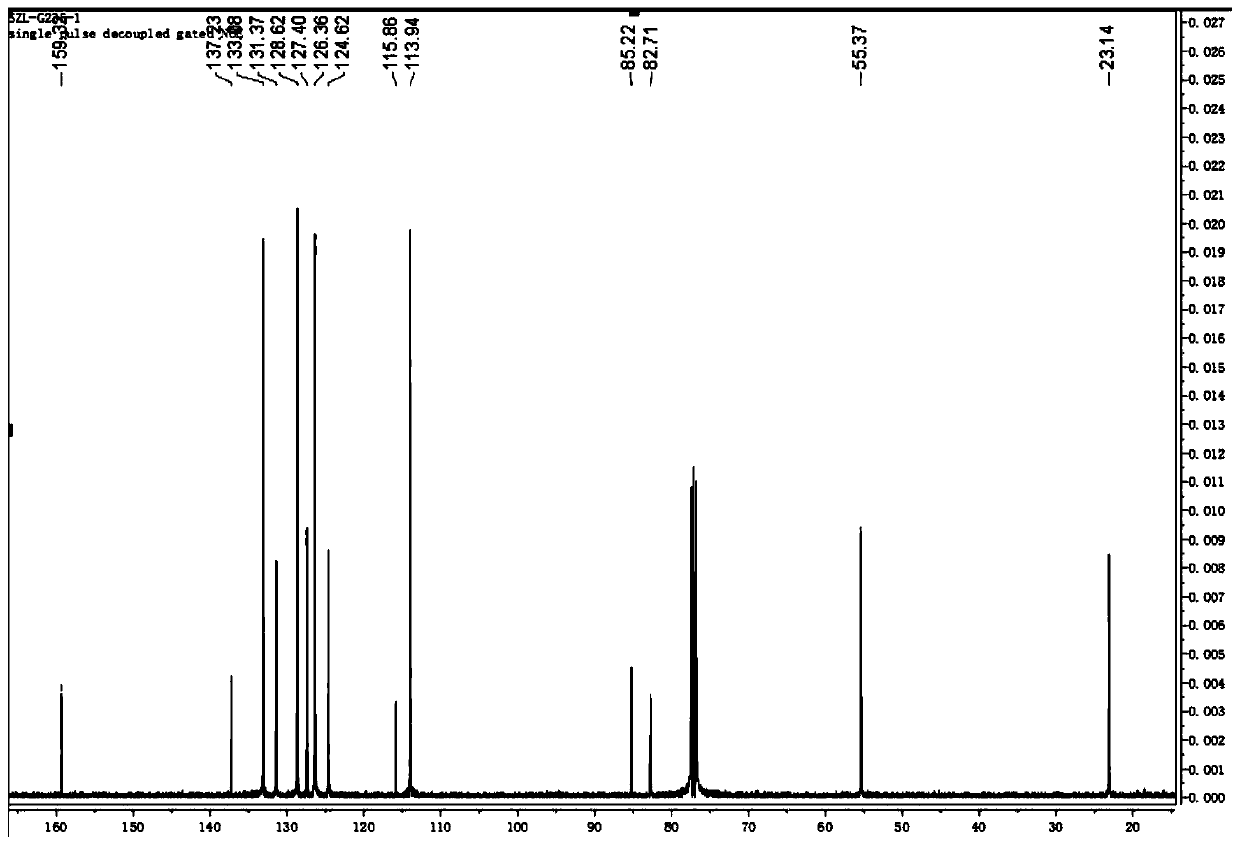
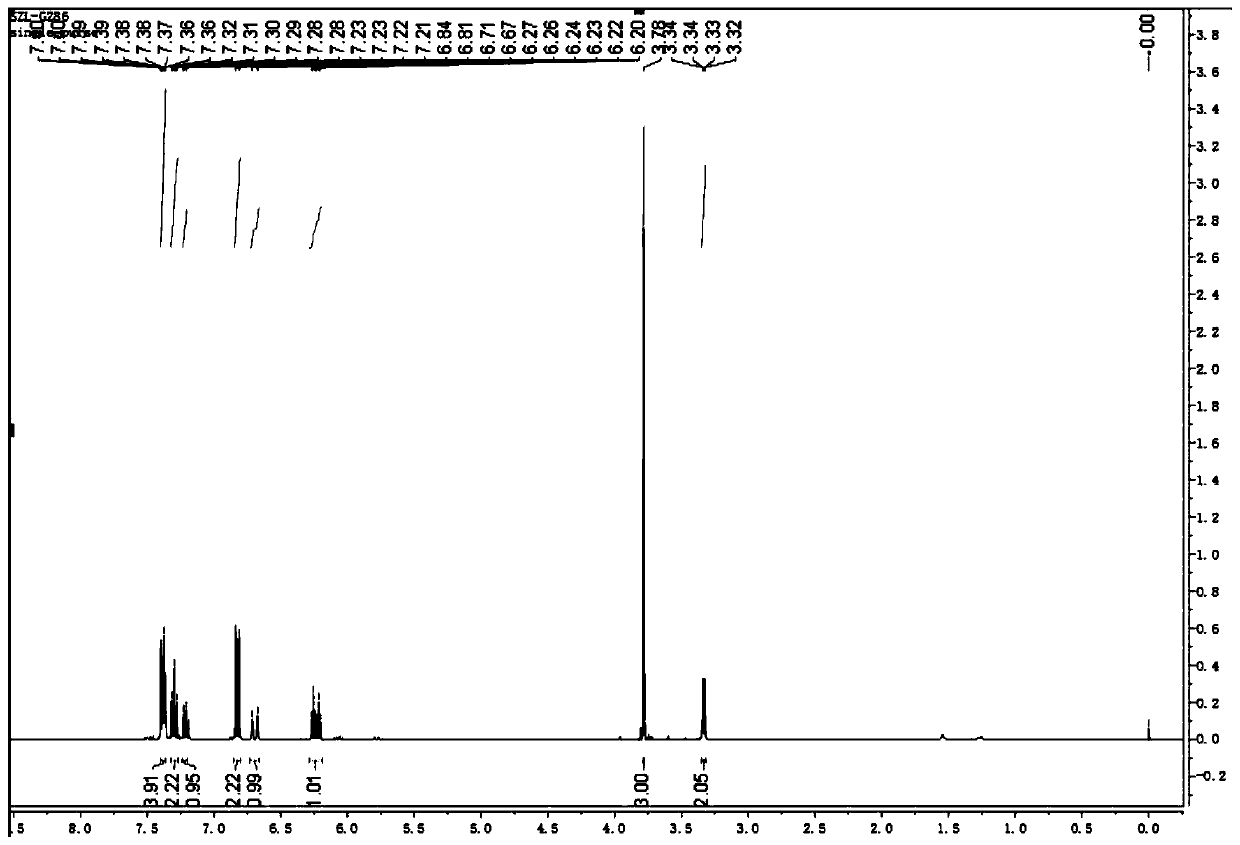
[0039] (2) After TLC monitors that the reaction is complete, the reaction solution is extracted, and the solvent is removed by a vacuum rotary evaporator, and the product is separated by thin-layer chromatography. ,like figure 1 , 2 As shown, the product was determined to be 1-methoxy-4-(5-phenylpent-4-en-1-yn-1-yl)benzene with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com