Method for preparing triphenylamine derivative
A technology of derivatives and triphenylamine, which is applied in the field of preparation of triphenylamine derivatives, can solve problems such as non-compliance with green chemistry, difficulty in separation process, complex reaction process, etc., and achieve the effect of reducing reaction time, optimizing process, and improving separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The technical solutions of the present invention will be described in detail below in conjunction with specific embodiments, but the protection scope of the present invention is not limited thereto.
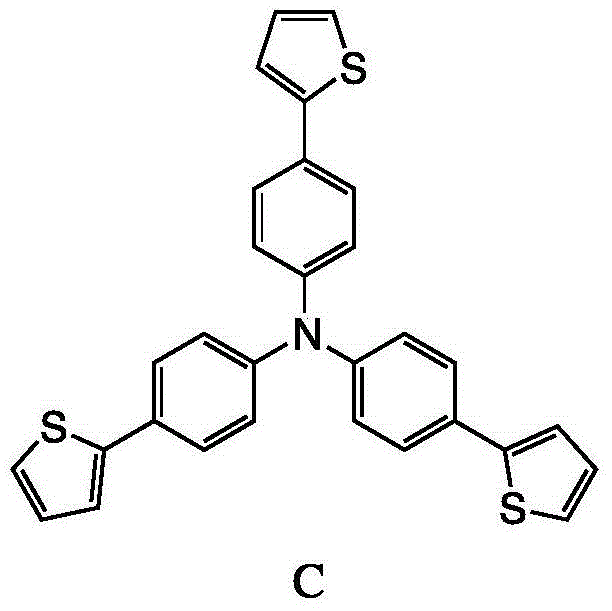
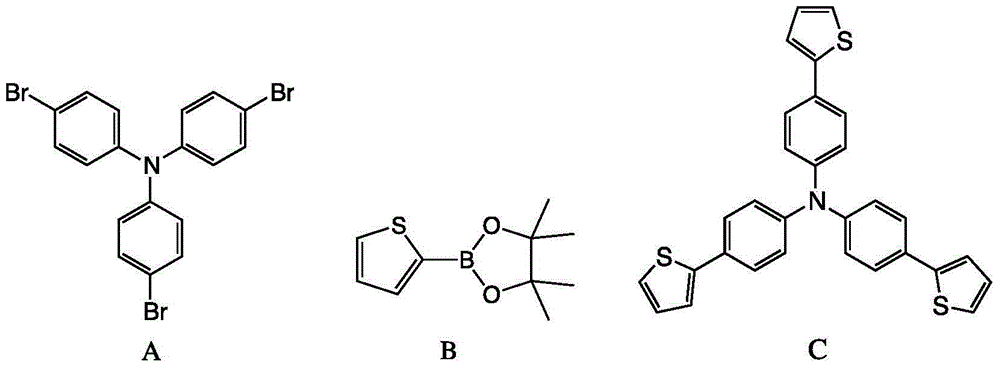
[0024] Compound A process route
[0025]
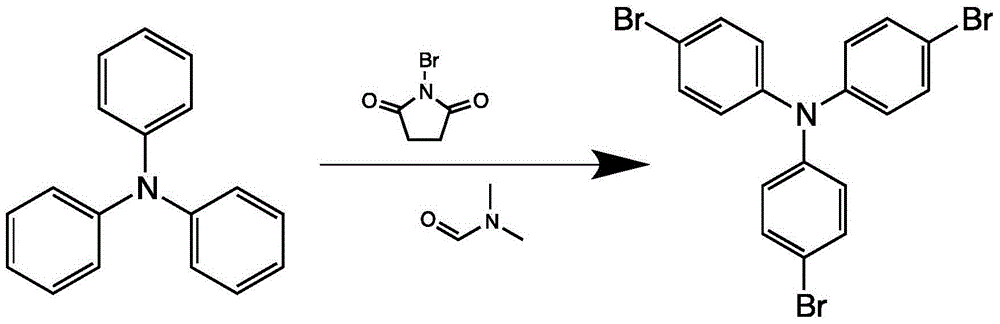
[0026] At 0°C, N-bromosuccinimide (24.0 g, 134.6 mmol), triphenylamine (10.0 g, 40.8 mmol), and dimethylformamide (120 mL) were added into a two-necked flask. The mixed solution was warmed up to room temperature and stirred for 12 hours. After the reaction was completed, 100 mL of water was added to quench the reaction, extracted with 150 mL of dichloromethane, and the organic phase was washed with 150 mL of saturated saline for three times, then dried with anhydrous magnesium sulfate, and the filtrate was filtered and distilled under reduced pressure. The solid obtained by distillation under reduced pressure was separated and purified by column chromatography, and the eluent was hexane, CH 2 Cl 2 A mixed solvent with a volu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com