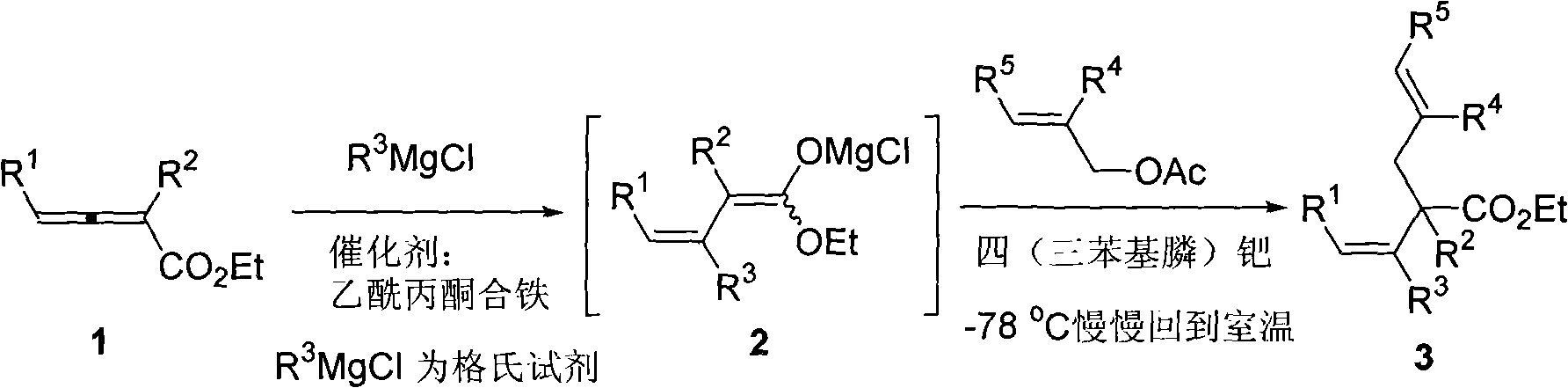
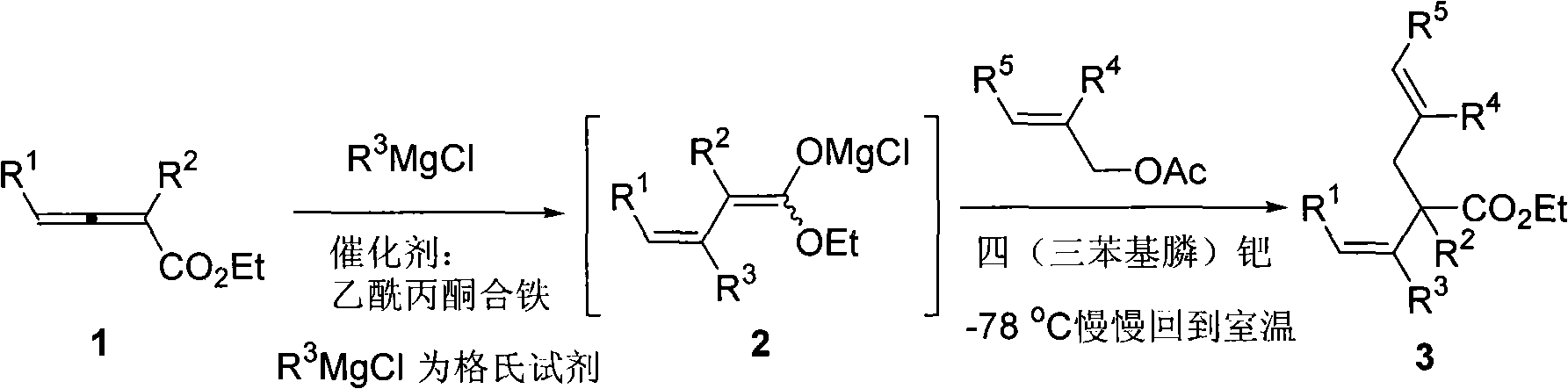
Method for synthesizing cis-alpha-allyl-beta, gamma-unsaturated carboxylic acid ester
A technology of allyl acetate and allyl, which is applied in the direction of carboxylate preparation, chemical instruments and methods, organic chemistry methods, etc., can solve the problems such as insufficient synthesis methods, and achieve high regio and stereoselectivity, high Stereoselective, easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Under nitrogen, ferric acetylacetonate (0.0072 g, 0.02 mmol), ethyl 2-methyl-4-phenyl-2,3-butadienoate (0.0805 g, 0.4 mmol) and Toluene (5 ml) was cooled to minus 78 degrees, and a solution of methylmagnesium chloride in tetrahydrofuran (0.4 ml, 3 equivalents, 1.2 mmol) was added dropwise to the system. After the addition was completed and reacted for 1 hour, tetrakis(triphenylphosphine) palladium (0.0225 g, 0.02 mmol) and allyl acetate (0.22 ml, d=0.928 g / ml, 0.2000 g, 2 mmol, 5 Equivalent) to react under stirring for 1. hour, naturally return to room temperature and react overnight, cool to zero and add dropwise saturated ammonium chloride solution to quench the reaction. Naturally warmed to room temperature, added water, extracted with ether, washed once with 1% hydrochloric acid, saturated sodium bicarbonate, and saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and flash column chromatography gave 2-allyl-2 , 0.0691 g of ethyl 3-dimethy...
Embodiment 2
[0024] According to the method described in Example 1, the difference is that the substrate and reagent used are: iron acetylacetonate (0.0073 grams, 0.02 mmol), 2-methyl-4-(4'-fluorophenyl)-2, Ethyl 3-butadienoate (0.0892 g, 0.4 mmol), tetrakis(triphenylphosphine) palladium (0.0230 g, 0.02 mmol) and allyl acetate (0.22 ml, d=0.928 g / ml, 0.2000 grams, 2 mmoles, 5 equivalents) to get 0.0817 grams of 2-allyl-2,3-dimethyl-4-(4'-fluorophenyl)-3(Z)-butenoic acid ethyl ester, product The rate is 74%. The product is a colorless liquid.
[0025] 1 H NMR (300MHz, CDCl 3 )δ7.10-7.01 (m, 2H), 6.98-6.88 (m, 2H), 6.41 (s, 1H), 5.75-5.58 (m, 1H), 5.08-4.95 (m, 2H), 3.66 (dq, J 1 =10.8Hz,J 2 =7.2Hz, 1H), 3.56(dq, J 1 =10.8Hz,J 2 =7.2Hz, 1H), 2.50-2.32(m, 2H), 1.91(d, J=1.2Hz, 3H), 1.27(s, 3H), 1.08(t, J=7.2Hz, 3H); 13 C NMR (CDCl 3 , 75MHz) δ175.2, 161.4 (d, J=244Hz), 139.7, 134.1 (d, J=3.1Hz), 133.9, 130.5 (d, J=7.9Hz), 126.8, 118.2, 114.4 (d, J= 21.8Hz), 60.3, 50.0, 42.8, 23.1, ...
Embodiment 3
[0027] According to the method described in Example 1, the difference is that the substrate and reagent used are: iron acetylacetonate (0.0070 g, 0.02 mmol), 2-methyl-4-(4'-chlorophenyl)-2, Ethyl 3-butadienoate (0.0959 g, 0.4 mmol), tetrakis(triphenylphosphine) palladium (0.0230 g, 0.02 mmol) and allyl acetate (0.22 ml, d=0.928 g / ml, 0.2000 grams, 2 mmoles, 5 equivalents) get 0.0770 grams of 2-allyl-2,3-dimethyl-4-(4'-chlorophenyl)-3(Z)-butenoic acid ethyl ester, produce The rate is 66%. The product is a colorless liquid.
[0028] 1 H NMR (300MHz, CDCl 3 )δ7.22(d, J=8.0Hz, 2H), 7.03(d, J=8.0Hz, 2H), 6.40(s, 1H), 5.75-5.58(m, 1H), 5.06-4.96(m, 2H ), 3.73-3.53(m, 2H), 2.50-2.30(m, 2H), 1.91(s, 3H), 1.26(s, 3H), 1.08(t, J=7.2Hz, 3H); 13 C NMR (CDCl 3 , 75MHz) δ175.1, 140.0, 136.7, 133.8, 132.2, 130.2, 127.7, 126.7, 118.3, 60.4, 50.1, 42.7, 23.1, 13.9; IR (neat, cm -1 ) 2979, 1730, 1640, 1593, 1488, 1230, 1091; MS (API-ES, m / z) 317.0 ((M( 37 Cl)+Na + ), 2.51), 315.1((M( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com