Preparation method of truxene-based star-like compound
A technology of a compound, dibromoindanal, which is applied in the field of preparation of star compounds, can solve the problems of complex reaction, many synthesis steps, and low yield, and achieve good reaction selectivity, simple synthesis route, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
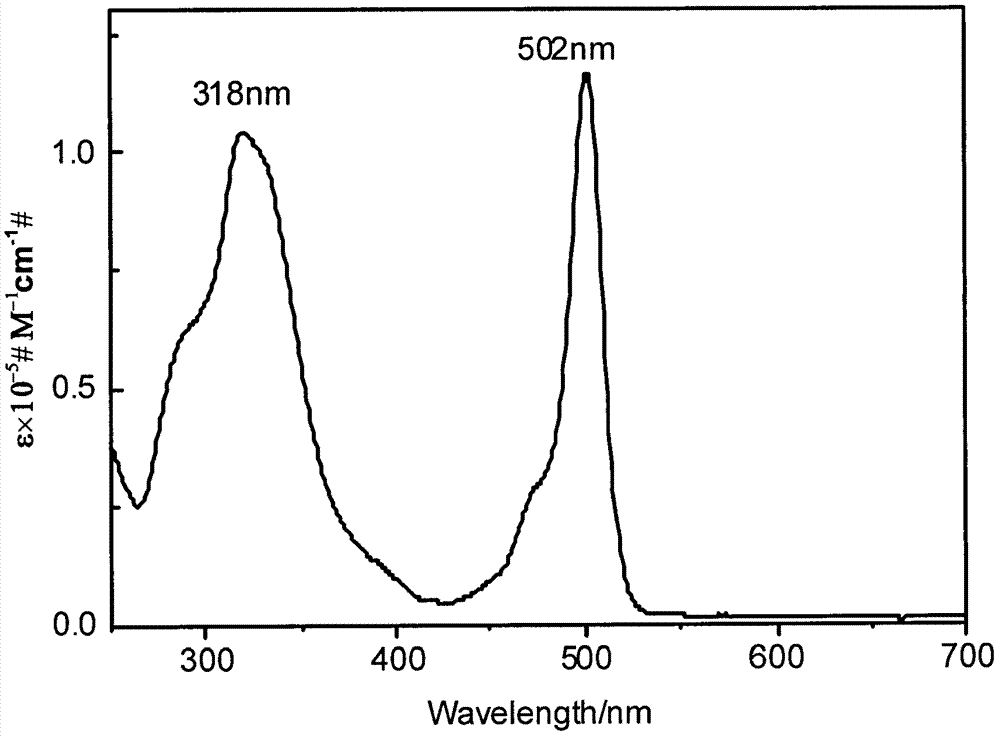
[0030] Dissolve 2,7-dibromotriindaldehyde (0.7mmol, 605.49mg), BODIPY borate derivative (0.7mmol, 315.10mg), anhydrous sodium carbonate (12.64mmol, 1.34g) under argon atmosphere in tetrahydrofuran (60 mL), and a mixture of methanol and water (CH 3 OH / H 2 O=5mL / 5mL), add catalyst Pd (PPh 3 ) 4 (0.07mmol, 77.39mg), heated to reflux for 24 hours. After the reaction, the organic matter was extracted with dichloromethane and washed with saturated ammonium chloride solution. The organic layers were combined and dried over anhydrous sodium sulfate. Analysis, separation and purification yielded 233.25 mg of product TB (yield 30.07%). UV-vis (CH 2 Cl 2 ), λ max / nm[ε×10 -5 / (L·mol -1 cm -1 )]: 318 (0.192), 502 (0.219) (with figure 1 ); 1 H NMR (CDCl 3 , 600MHz, ppm) δ 10.15(s, 2H), 8.44-8.48(m, 1H), 8.25-8.28(m, 1H), 7.92-7.96(m, 3H), 7.44(d, J=6Hz, 2H) , 7.61(s, 1H), 6.02(s, 2H), 2.92-3.03(m, 6H), 2.09-2.24(m, 6H), 1.54(d, J=12Hz, 6H), 0.44-0.52(m, 26H).Emission Wavele...
Embodiment 2
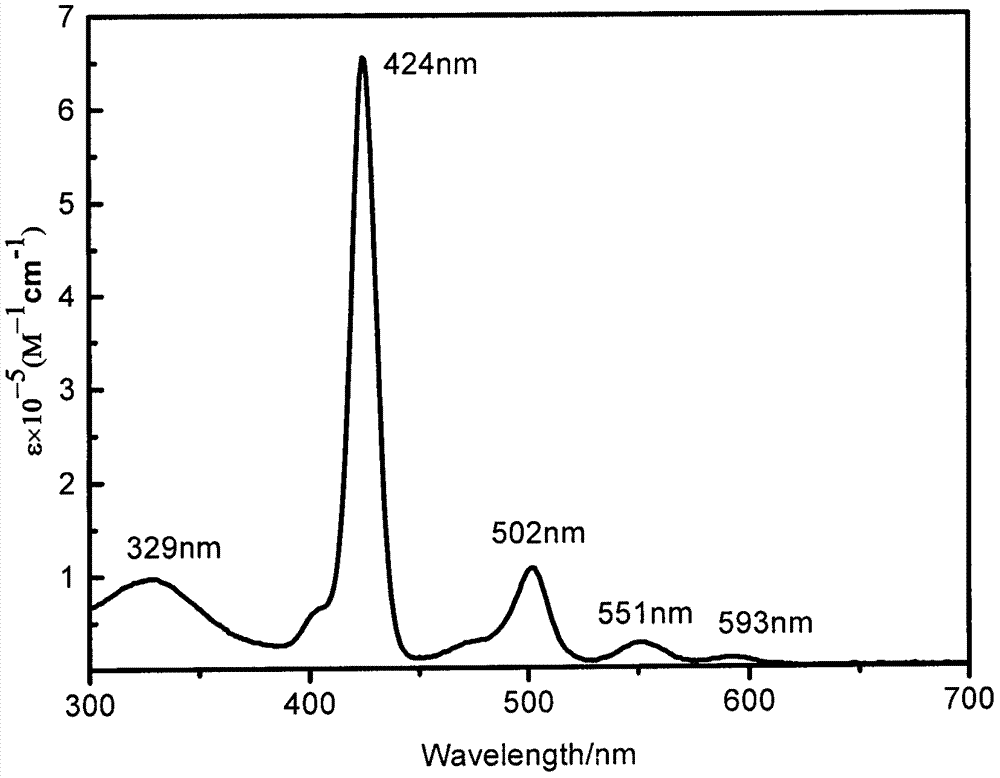
[0032] Compound TB (0.15mmol, 166.22mg) and porphyrin derivative (0.15mmol, 144.36mg) were dissolved in dry tetrahydrofuran (40mL) under argon atmosphere, anhydrous sodium carbonate (2.7mmol, 286.17mg) was added, And add catalyst Pd(PPh 3 ) 4 (0.015mmol, 17.33mg), then add methanol and water mixture (CH 3 OH / H 2 O=1mL / 1 mL), the reaction system was reacted under reflux conditions for 24 hours. After the reaction, the organic matter was extracted with dichloromethane and washed with saturated ammonium chloride solution. After the organic layers were combined, they were dried over anhydrous sodium sulfate and reduced pressure The solvent was distilled off, and the product TBP was separated and purified by silica gel column chromatography with dichloromethane-petroleum ether as the eluent to obtain 73.5 mg (yield: 26.4%). UV-vis (CH 2 Cl 2 ), λ max / nm[ε×10 -5 / (L·mol -1 cm -1 )]: 329 (0.97319), 424 (6.55283), 502 (1.07515), 551 (0.26879), 593 (0.10195) (with figure 2 ...
Embodiment 3
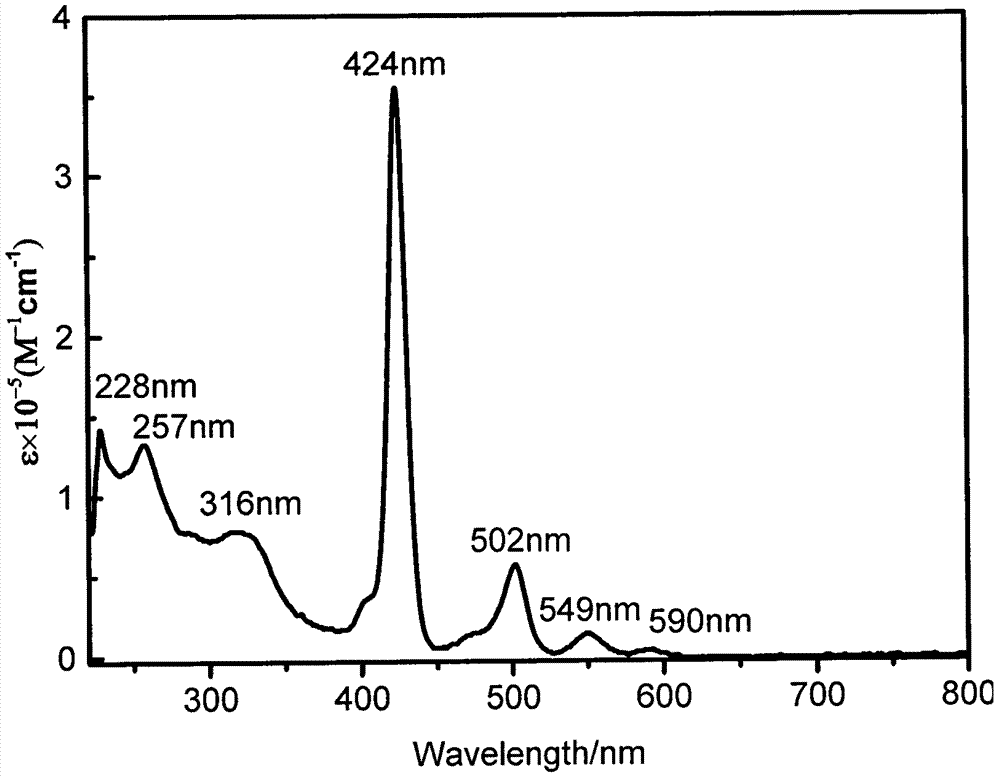
[0034] The compound TBP (0.0217mmol, 40mg), fullerene (0.109mmol, 78.20mg) and sarcosine (0.217mmol, 19.33mg) were dissolved in dry toluene (30mL), and heated under reflux under argon for 12 hours After the reaction is over, the solvent is distilled off under reduced pressure and is separated and purified by silica gel column chromatography with dichloromethane-petroleum ether as an eluent to obtain the product TBP-C 60 It was 34.72 mg (61.51% yield). UV-vis (CH 2 Cl 2 ), λ max / nm[ε×10 -5 / (L·mol -1 cm -1 )]: 257 (1.32673), 316 (0.78043), 424 (3.56396), 502 (0.57231), 549 (0.14308), 590 (0.039020) (attached image 3 ); 1 H NMR (CDCl 3 , 600MHz, ppm) δ9.04-9.06(m, 4H), 9.00(t, J=3.0Hz, 2H), 8.96(s, 2H), 8.71-8.73(m, 1H), 8.53(t, J= 3.6Hz, 2H), 8.42-8.46(m, 1H), 8.29(s, 2H), 8.23(s, 2H), 8.09-8.14(m, 5H), 7.95(t, J=6.6Hz, 2H), 7.85(s, 1H), 7.74-7.80(m, 7H), 7.42-7.44(d, J=7.2Hz, 2H), 6.02(s, 2H), 5.05(s, 1H), 4.94-4.96(m, 1H), 4.21-4.22(m, 1H), 3.28-3.31(m, 2H), 3.04...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com