Conjugate microporous polymer based on BODIPY derivative and preparation method thereof
A conjugated microporous and polymer technology, applied in the field of conjugated microporous polymers and their preparation, to achieve good chemical and thermal stability, good porosity, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
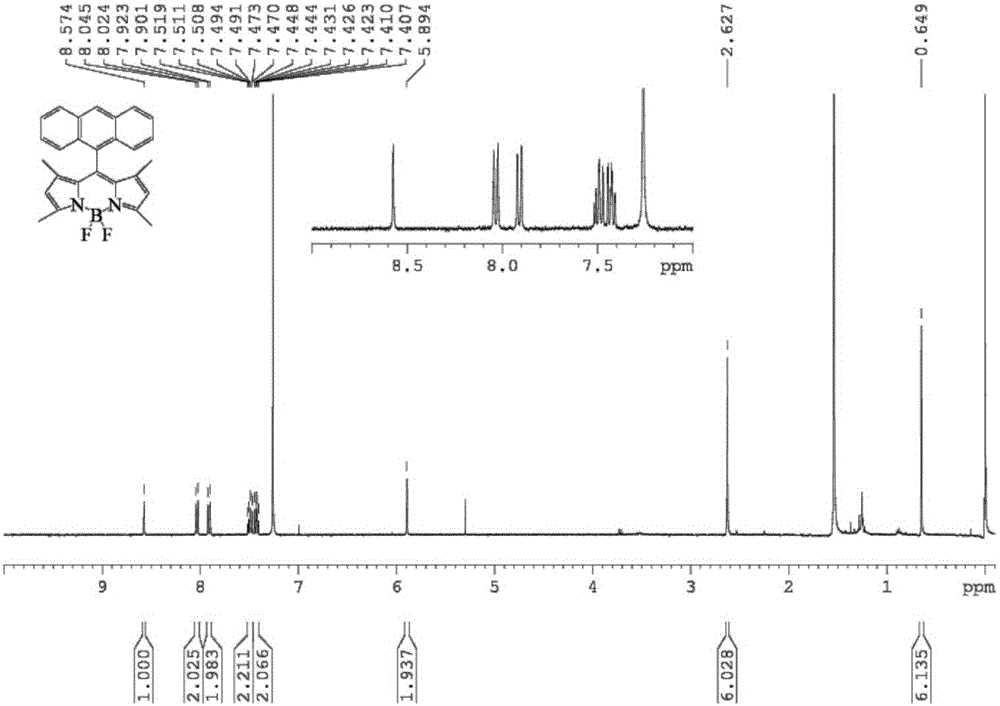
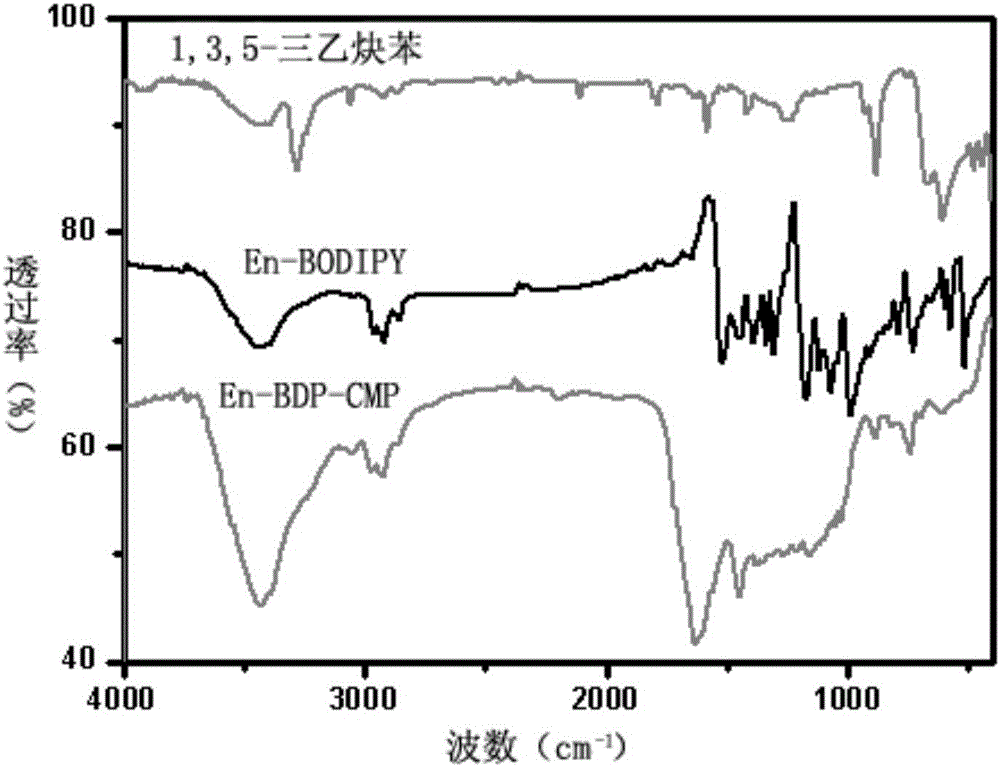
[0040] A conjugated microporous polymer based on BODIPY derivatives of the present invention, the conjugated microporous polymer uses 9-anthracene formaldehyde and 2,4-dimethylpyrrole as raw materials, and obtains anthracene-containing groups through multi-step reactions The BODIPY chromophore, and then the BODIPY chromophore containing anthracene group and 1,3,5-triethynylbenzene in tetrakis (triphenylphosphine) palladium (Pd (PPh 3 ) 4 ) and CuI catalyzed by the Sonogashira reaction. Its concrete preparation steps are as follows:
[0041] (1) Weigh 9-anthracene formaldehyde (4mmol, 825mg), dissolve 2,4-dimethylpyrrole (8mmol, 761mg) in dry dichloromethane (DCM), add 4 drops of trifluoroacetic acid (TFA), The color of the solution changed from initial yellow to black, and the reaction was carried out under nitrogen protection for 12 h at room temperature. After the reaction was completed, dichlorodicyanoquinone (DDQ) (4 mmol, 908 mg) was added and stirred for 3 h, at this ...
Embodiment 2
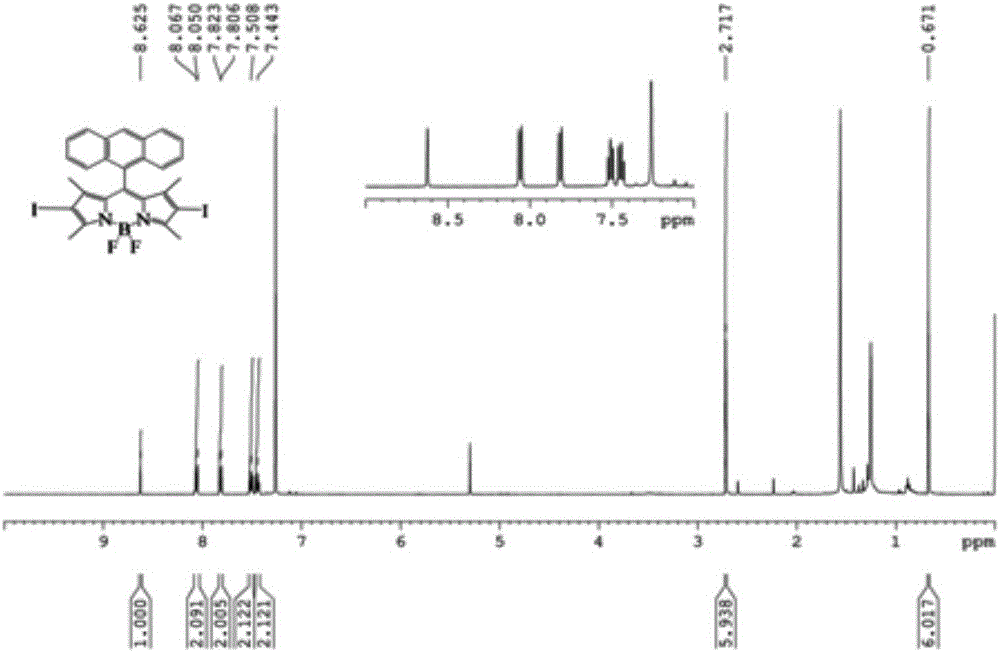
[0049] A conjugated microporous polymer based on BODIPY derivatives of the present invention, the conjugated microporous polymer uses 9-anthracene formaldehyde and 2,4-dimethylpyrrole as raw materials, and obtains anthracene-containing groups through multi-step reactions The BODIPY chromophore, and then the BODIPY chromophore containing anthracene group with 1,3,5-triethynylbenzene in Pd(PPh 3 ) 4 It is prepared by Sonogashira reaction under the catalysis of CuI. Its concrete preparation steps are as follows:
[0050] (1) Weigh 9-anthracene formaldehyde (4mmol, 825mg), dissolve 2,4-dimethylpyrrole (10mmol, 951mg) in dry dichloromethane, add 4 drops of trifluoroacetic acid, the solution color changes from initial yellow Turned black, nitrogen protection reaction at room temperature for 18h. After the reaction was completed, dichlorodicyanoquinone (4 mmol, 908 mg) was added and stirred for 4 h. At this time, the color of the solution was slightly green. Add 7ml of N,N-diisop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com