Quinazoline derivative and preparation method and application thereof
A derivative, quinazoline technology, applied in drug combinations, antineoplastic drugs, organic chemistry, etc., to achieve the effects of less by-products, high product purity, and wide substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of the present invention may also include various post-treatment methods commonly used in the art, such as filtration, purification and other steps. The present invention has no special limitation on the post-processing steps. For example, in the present invention, the mixture obtained after the contact reaction can be filtered to remove the filter residue, and then the obtained filtrate is concentrated and purified by column chromatography.
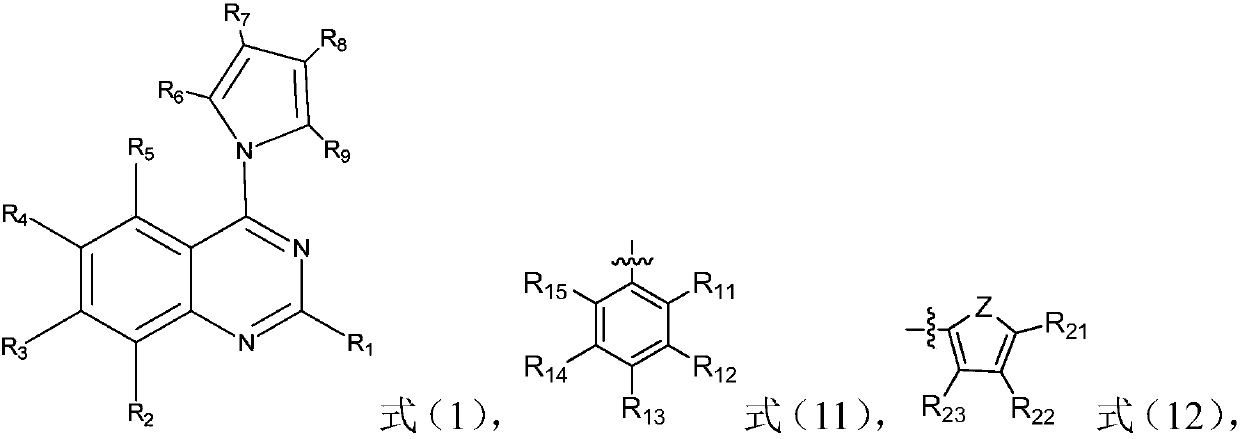
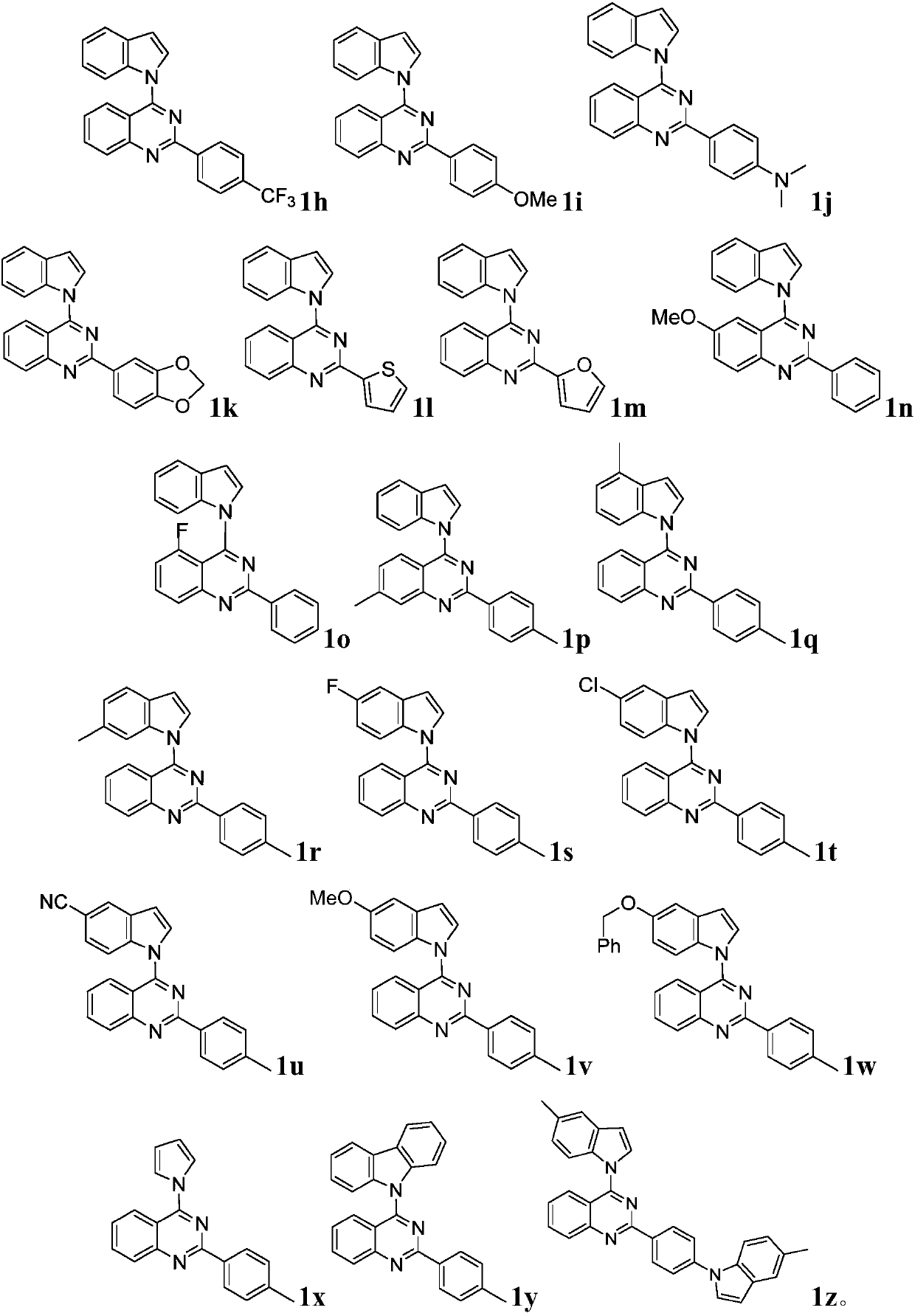
[0053] The third aspect of the present invention provides quinazoline derivatives prepared by the method described in the aforementioned second aspect.
[0054] And, the fourth aspect of the present invention provides the use of the quinazoline derivatives described in the first and third aspects as antibacterial and / or antitumor agents.
[0055] In the present invention, only a small amount of catalyst is used to make the conversion rate of the reaction raw material reach 100%. Simultaneously, the reactio...
Embodiment 1
[0061] Embodiment 1: preparation compound 1a
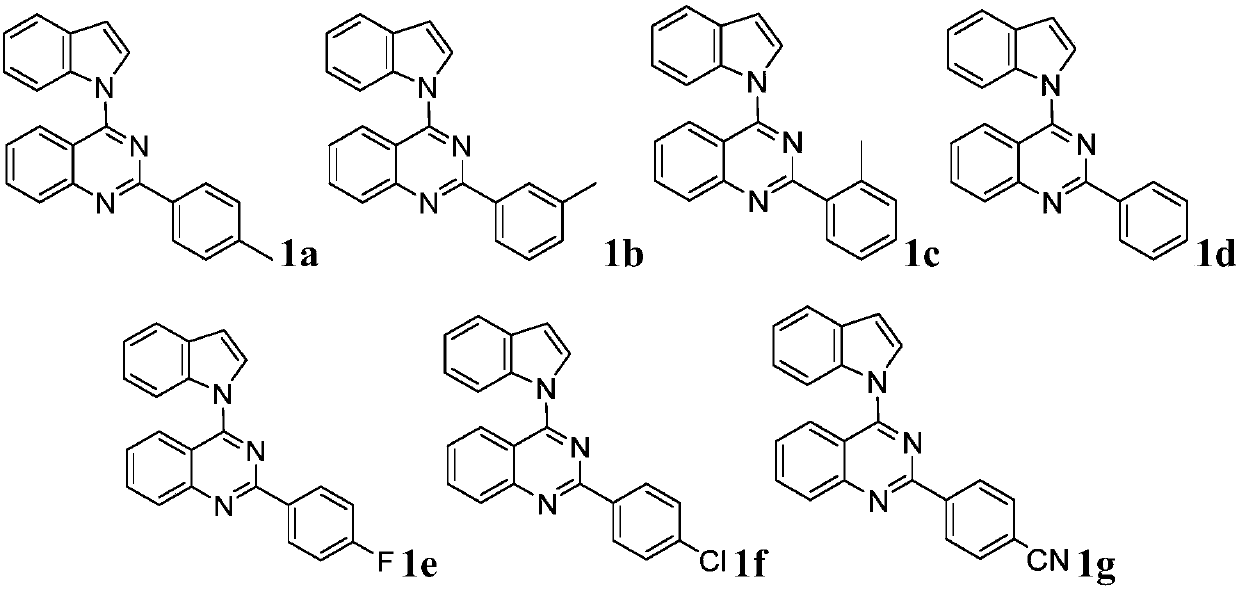
[0062] Under nitrogen protection, 4-(4-p-methylbenzenesulfonyl)-2-p-tolylquinazoline (0.20mmol), indole (0.22mmol), palladium trifluoroacetate (0.01mmol), 2-bicyclohexyl Phosphine-2',6'-dimethoxybiphenyl (0.02mmol), potassium carbonate (0.40mmol) and 2.0mL toluene were added to the reaction flask, and the reaction was carried out on a magnetic stirrer at 110°C for 6h, and the reaction was detected by TLC. . After the reaction was completed, the catalyst was removed by suction filtration through a sand core funnel equipped with silica gel, and the obtained filtrate was separated by silica gel column chromatography with a volume ratio of petroleum ether and ethyl acetate of 50:1 to obtain the pure product 4-(N-indole Indolyl)-2-p-tolylquinazoline is compound 1a. Yield: 83%.
[0063]Compound 1a is a white solid with a melting point of 167-168°C. 1 H NMR (400MHz, CDCl 3 )δ2.44(s,3H),6.83(d,J=3.2Hz,1H),7.26-7.34(m,4H),7.53(t,J=7.6...
Embodiment 2
[0066] Embodiment 2: preparation compound 1b
[0067] The present embodiment adopts the method similar to embodiment 1 to carry out, the difference is, the present embodiment adopts 4-(4-p-methylbenzenesulfonyl)-2-m-tolylquinazoline (0.20mmol) to replace the embodiment 4-(4-p-Tolylsulfonyl)-2-p-tolylquinazoline (0.20 mmol) in 1. All the other are the same as in Example 1.
[0068] The product 4-(N-indolyl)-2-m-tolylquinazoline was obtained, namely compound 1b. Yield: 85%.
[0069] Compound 1b is a white solid with a melting point of 164-165°C. 1 H NMR (400MHz, CDCl 3 )δ2.46(s,3H),6.81(d,J=3.4Hz,1H),7.24-7.33(m,3H),7.40(t,J=7.6Hz,1H),7.49(t,J=8.2 Hz,1H),7.69-7.72(m,2H),7.84(t,J=8.4Hz,1H),7.96(d,J=7.6Hz,1H),8.04(d,J=8.0Hz,1H), 8.13(d,J=8.4Hz,1H),8.43-8.47(m,2H). 13 C NMR (100MHz, CDCl 3 )δ21.6, 106.7, 113.6, 117.4, 121.2, 122.2, 123.6, 125.5, 126.0, 127.2, 128.55, 128.6, 129.26, 129.3, 130.2, 131.8, 134.0, 136.5, 1357.6, 130.10.3, 15
[0070] IR(KBr):2916,1616,1413,1130...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com