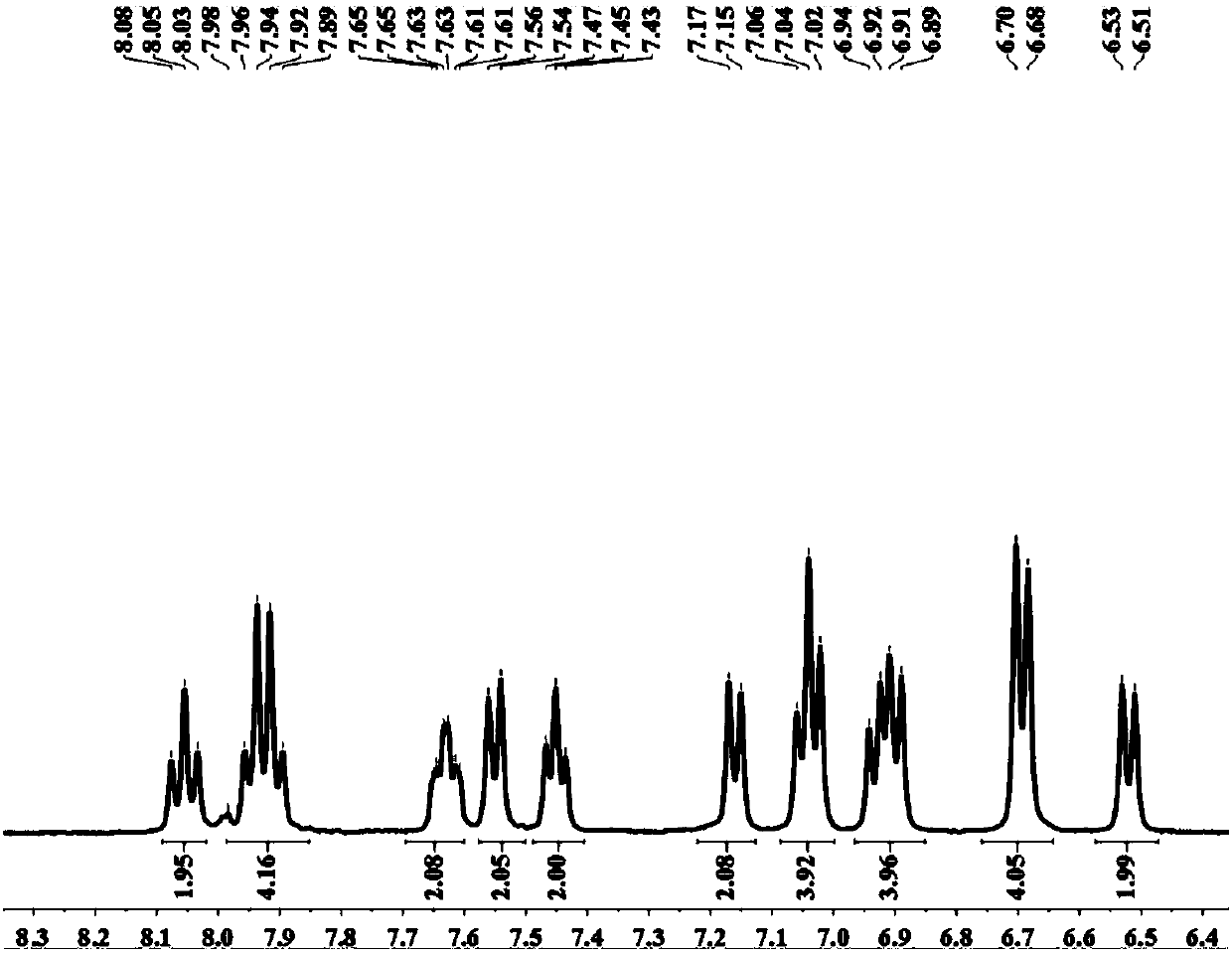
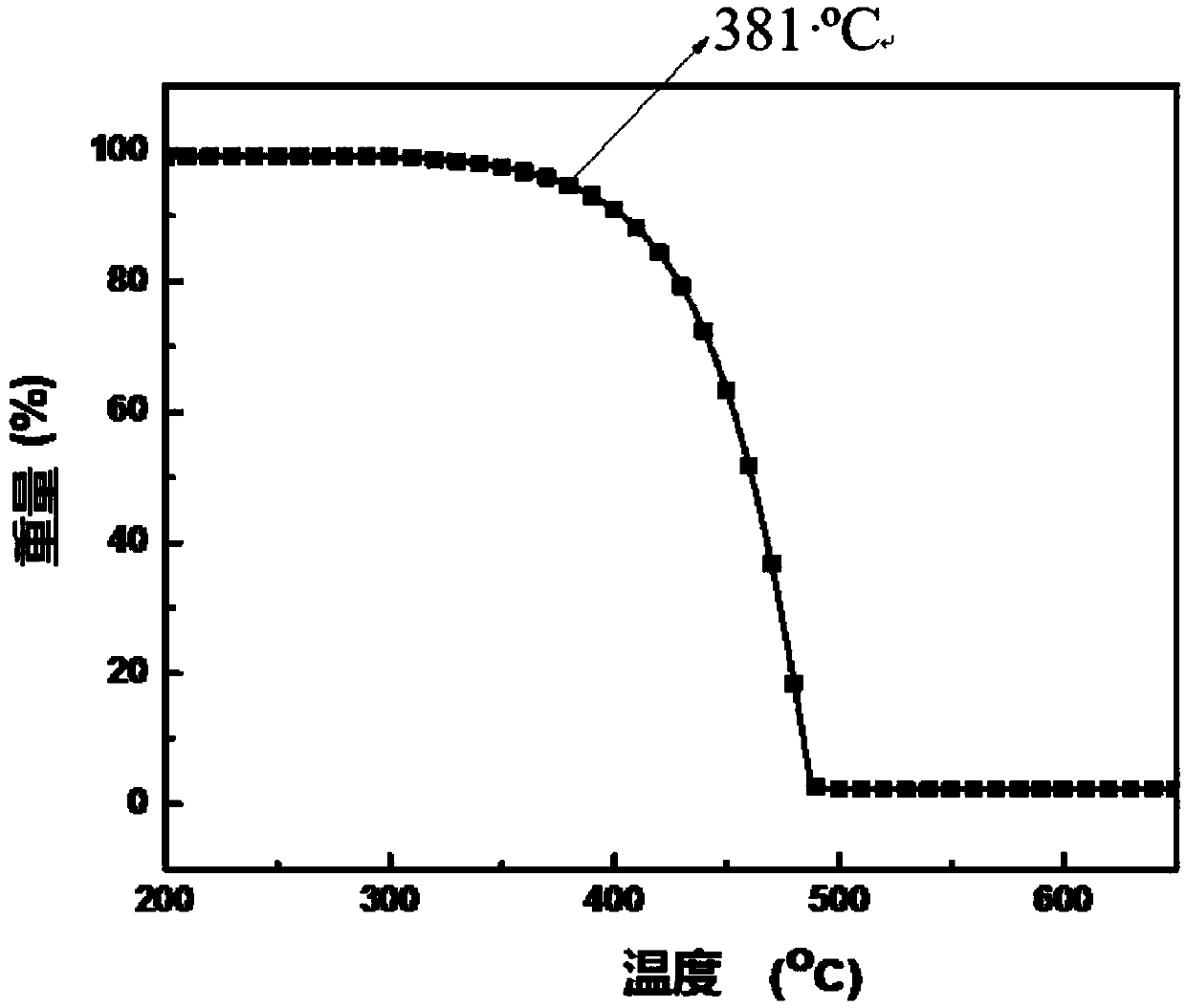
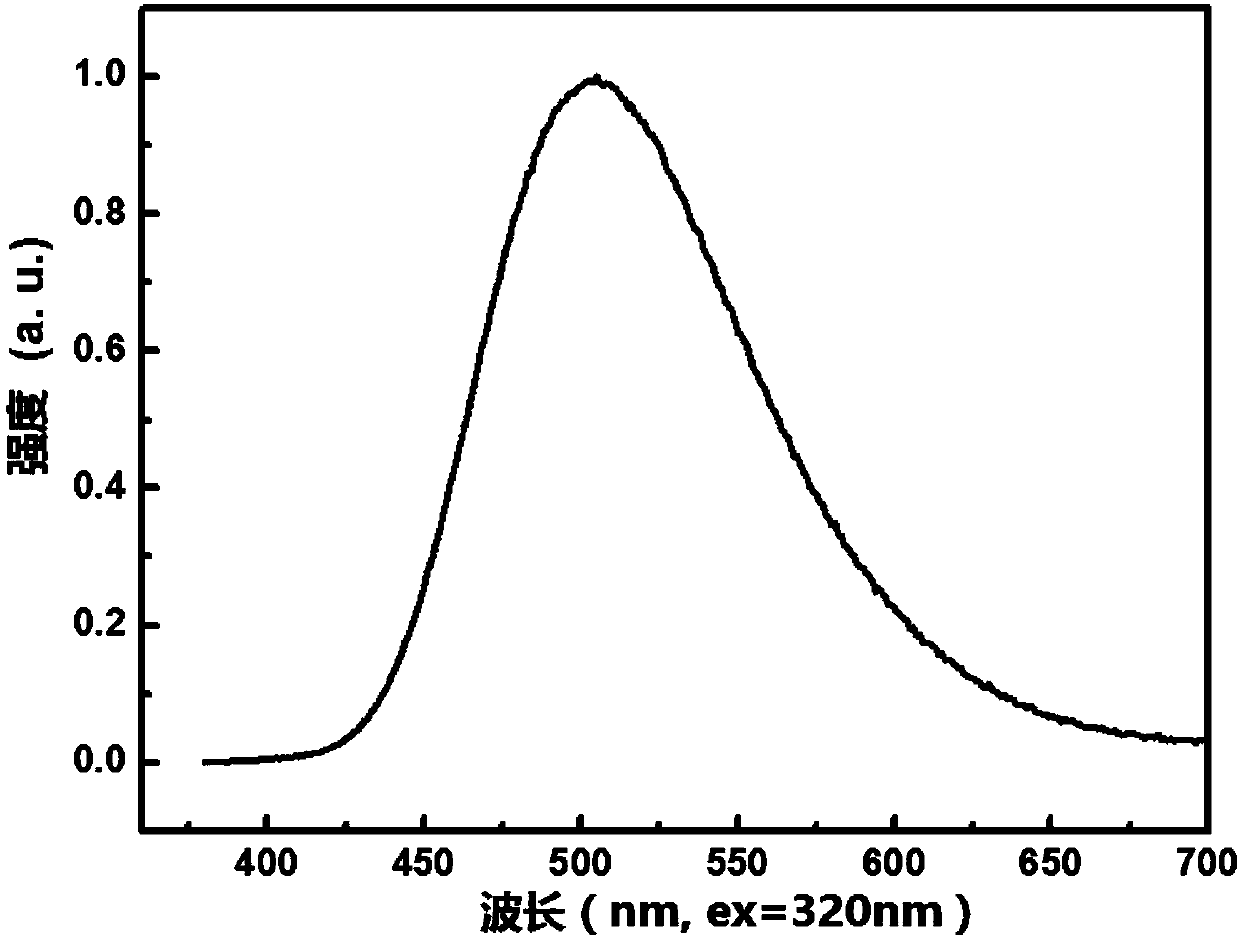
Blue fluorescent material and preparation method thereof
A blue fluorescent and precursor technology, applied in the field of blue fluorescent materials and their preparation, can solve the problems of poor thermal stability of blue fluorescent materials, and achieve the effect of reducing non-radiative deactivation and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing a blue light fluorescent material, comprising the following steps:
[0028] S1. Synthesis of precursor I: In a mixed solution containing potassium carbonate and toluene, add 4-cyanophenylboronic acid, 1,4-dibromobenzene, tetrakis(triphenylphosphine)palladium, and then heat at 80°C Reflux reaction for 27 hours, after extraction and purification, the solid 4-bromo-4'-cyanobiphenyl was collected to obtain the precursor I; wherein the 4-cyanophenylboronic acid, 1,4-dibromobenzene , The molar ratio of tetrakis(triphenylphosphine)palladium is 41:46:0.2.
[0029] S2. Synthesis of precursor II: In anhydrous 1,4-dioxane solution, add precursor I, bis(pinacolate) diboron, potassium acetate and 1,1'-bis(diphenyl Phosphine) ferrocene dichloride palladium dichloromethane complex, heated to reflux at 80°C for 25 hours, purified by extraction, collected solid 4'-cyano-[1,1'-biphenyl]-4- Borate ester, that is, to obtain precursor II; wherein, in molar ratio, th...
Embodiment 2
[0033] A method for preparing a blue light fluorescent material, comprising the following steps:
[0034] S1. Synthesis of precursor I: In a mixed solution containing potassium carbonate and toluene, add 4-cyanophenylboronic acid, 1,4-dibromobenzene, tetrakis(triphenylphosphine)palladium, and then heat at 75°C Reflux reaction for 24 hours, after extraction and purification, the solid 4-bromo-4'-cyanobiphenyl was collected to obtain the precursor I; wherein the 4-cyanophenylboronic acid, 1,4-dibromobenzene , The molar ratio of tetrakis(triphenylphosphine)palladium is 40:45:0.2.
[0035] S2. Synthesis of precursor II: In anhydrous 1,4-dioxane solution, add precursor I, bis(pinacolate) diboron, potassium acetate and 1,1'-bis(diphenyl Phosphine) ferrocene dichloride palladium dichloromethane complex, heated to reflux at 88°C for 22 hours, purified by extraction, collected solid 4'-cyano-[1,1'-biphenyl]-4- Borate ester, that is, to obtain precursor II; wherein, in molar ratio, th...
Embodiment 3
[0039] A method for preparing a blue light fluorescent material, comprising the following steps:
[0040] S1. Synthesis of precursor I: In a mixed solution containing potassium carbonate and toluene, add 4-cyanophenylboronic acid, 1,4-dibromobenzene, tetrakis(triphenylphosphine)palladium, and then heat at 85°C Refluxing reaction for 30 hours, after extraction and purification, solid 4-bromo-4'-cyanobiphenyl was collected to obtain precursor I; wherein the 4-cyanophenylboronic acid, 1,4-dibromobenzene , The molar ratio of tetrakis(triphenylphosphine)palladium is 42:47:0.3.
[0041] S2. Synthesis of precursor II: In anhydrous 1,4-dioxane solution, add precursor I, bis(pinacolate) diboron, potassium acetate and 1,1'-bis(diphenyl Phosphine) ferrocene dichloride palladium dichloromethane complex, heated and refluxed at 95°C for 28 hours, purified by extraction, collected solid 4'-cyano-[1,1'-biphenyl]-4- Borate ester, that is, to obtain precursor II; wherein, in molar ratio, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com