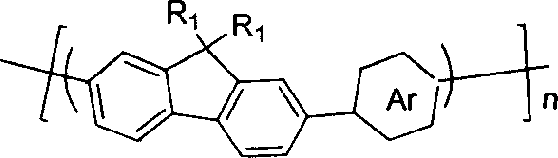
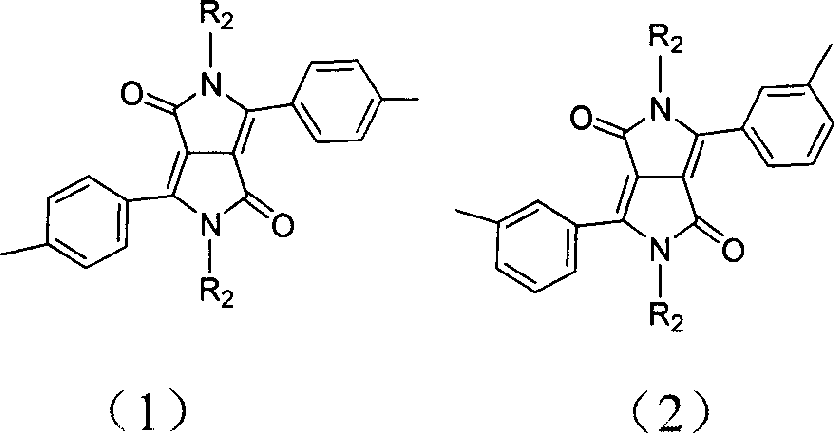
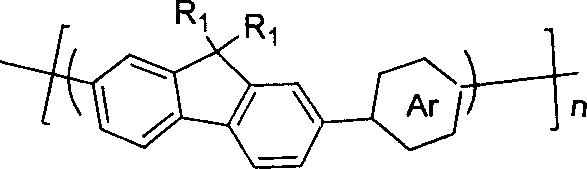
Pyrrolepyrrolidine-diones-fluorene copolymer electroluminescent material and its preparation method
A technology of pyrrolopyrrole dilidone and pyrrolopyrrole dilidone, which is applied in the field of pyrrolopyrrole dilidone-fluorene copolymer electroluminescent materials and its preparation, can solve fluorescence quenching, intensify molecular aggregation, and luminescence Low efficiency and other issues, to achieve the effect of broad absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of 3,6-bis(4-bromophenyl)pyrrolo[3,4c]pyrrole-1,4-dione (DPP1):
[0026] 4.6 g of metallic sodium (0.2 mol) and a catalytic amount of FeCl 3 Add 100ml of tert-amyl alcohol, keep the temperature at 90°C, stir and reflux for about 1h, until the sodium metal is completely dissolved. After cooling to about 50°C, 18.2 g of 4-bromobenzonitrile (0.1 mol) was added, and the temperature was raised to 90°C again. 8.08 g of diisopropyl succinate (0.04 mol) was dissolved in about 30 ml of tert-amyl alcohol, and slowly dropped into the reaction system in about 3 hours. Subsequently, the reaction suspension continued to reflux and stir at 90° C. for 20 h. The reaction mixture was cooled to 50°C, about 40ml of glacial acetic acid was slowly added, and refluxed for 4h. Filter the reaction mixture through a fritted funnel. The resulting solid was filtered and washed repeatedly with methanol and water until the solution was almost colorless. Drying under vacuum at 80°C...
Embodiment 2
[0030] Synthesis of pyrrolopyrrolidone-fluorene copolymer (P1): the reaction system uses high-purity N 2 emptying. 0.201g (0.3mmol) and 0.151g of 2,5-dioctyl-3,6-di(4-bromophenyl)pyrrolo[3,4c]pyrrole-1,4-dione prepared in Example 1 2,7-bis(trimethylene borate)-9,9-dihexylfluorene (F1) (0.3mmol), 0.69g K 2 CO 3 (5mmol) and 0.007g tetrakis (triphenylphosphine) palladium (Pd (PPh 3 ) 4 (0.006mmol) at N 2 Dissolve in a mixed solvent of 5mL tetrahydrofuran (THF) and 5mL water under protected conditions. Keep the temperature at 80°C and stir vigorously for 48h. Add 0.012g F1 (0.018mmol) to the reaction system, keep the temperature at 80°C, and stir for 6h; then add 0.008g of 2-bromo-9,9-dioctylfluorene (E) (0.018mmol), keep the temperature at 80°C, and stir for 6h . The reaction mixture was cooled and precipitated into methanol to give a solid. Filter, wash the solid with dilute hydrochloric acid, dissolve the solid again in chloroform, and precipitate in acetone. The prod...
Embodiment 3~9
[0032] The synthesis of pyrrolopyrrolidone-fluorene copolymer (P1): the preparation process is the same as in Example 2, the difference is to use NaHCO 3 , K 3 PO 4 , Na 2 CO 3 and Et 3 N as base instead of K 2 CO 3 , using different organic solvents and different solvent ratios, and different reaction times. Concrete alkali consumption, organic solvent and specific solvent proportion and reaction time are shown in Table 1 in each embodiment. The molecular weights of the obtained polymers are also different in each embodiment due to the difference in reaction conditions. The maximum photoluminescence wavelength λ of all the prepared copolymers in the film p1 It is 600-610nm; their luminescent wavelength is in the red light region, and they are good photoluminescent red light materials. The maximum electroluminescent wavelength λ of the copolymer in the device structure ITO / PEDOT / P1 / Ba / Al is 590-610nm, and their luminescent wavelength is in the red light region, so the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com