Preparation method and application of conjugated molecule based on fluoro-benzo-thiadiazole
A technology of benzothiadiazole and conjugated molecules is applied in the field of preparation of conjugated molecules, and can solve the problems of unstable structure, low light absorption rate and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
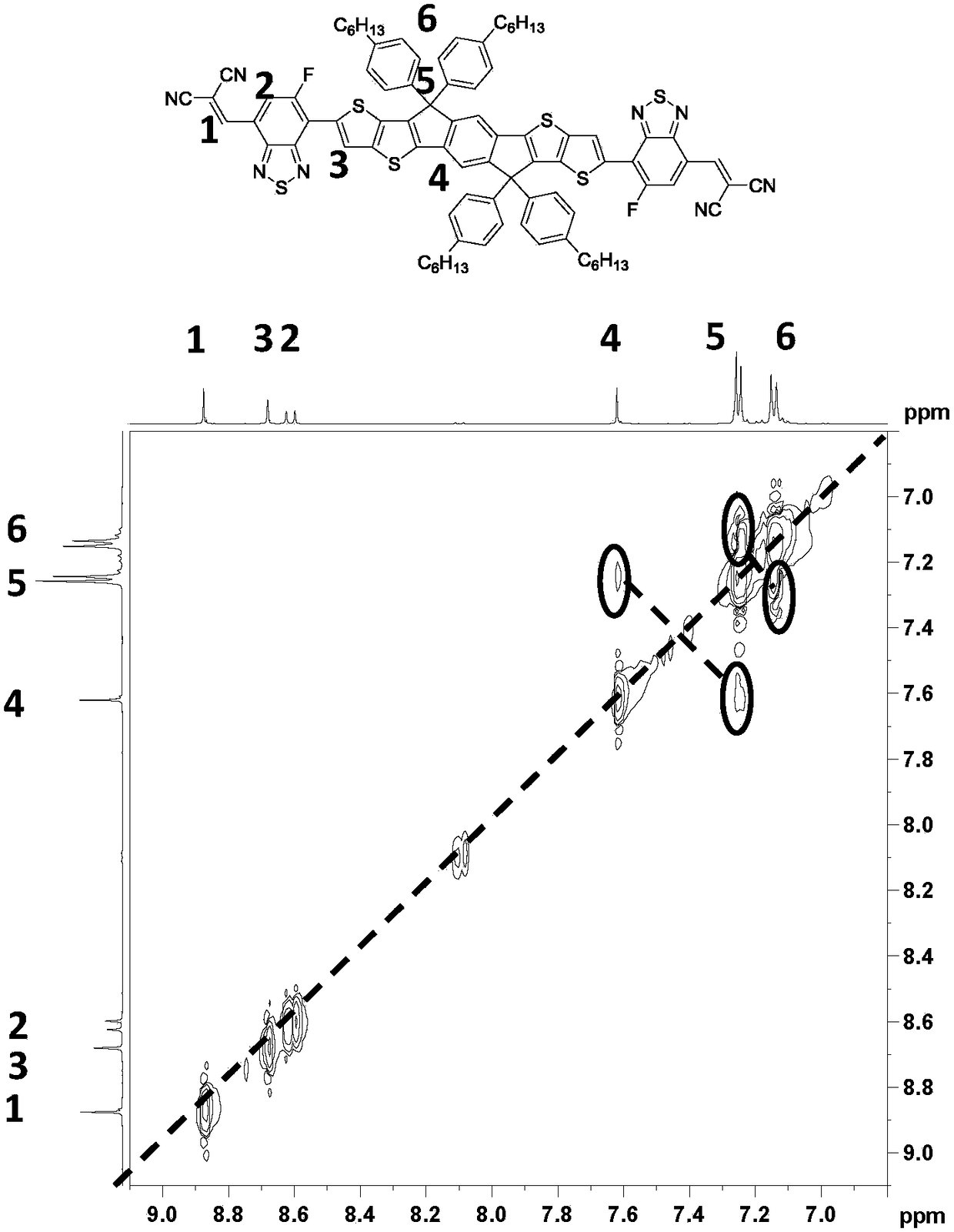
[0022] The embodiment of the present invention discloses a preparation method of a conjugated molecule based on fluorobenzothiadiazole, comprising the following steps: using toluene as a solvent, tetrakis(triphenylphosphine)palladium as a catalyst, and IDTT-2Sn and 2-((6-fluoro-7-ylbenzo[c][1,2,5]thiadiazole-4-yl)methylene)malononitrile is reacted at 90~110℃ to obtain Conjugated molecules of benzothiadiazoles. The reaction process is as follows:
[0023]
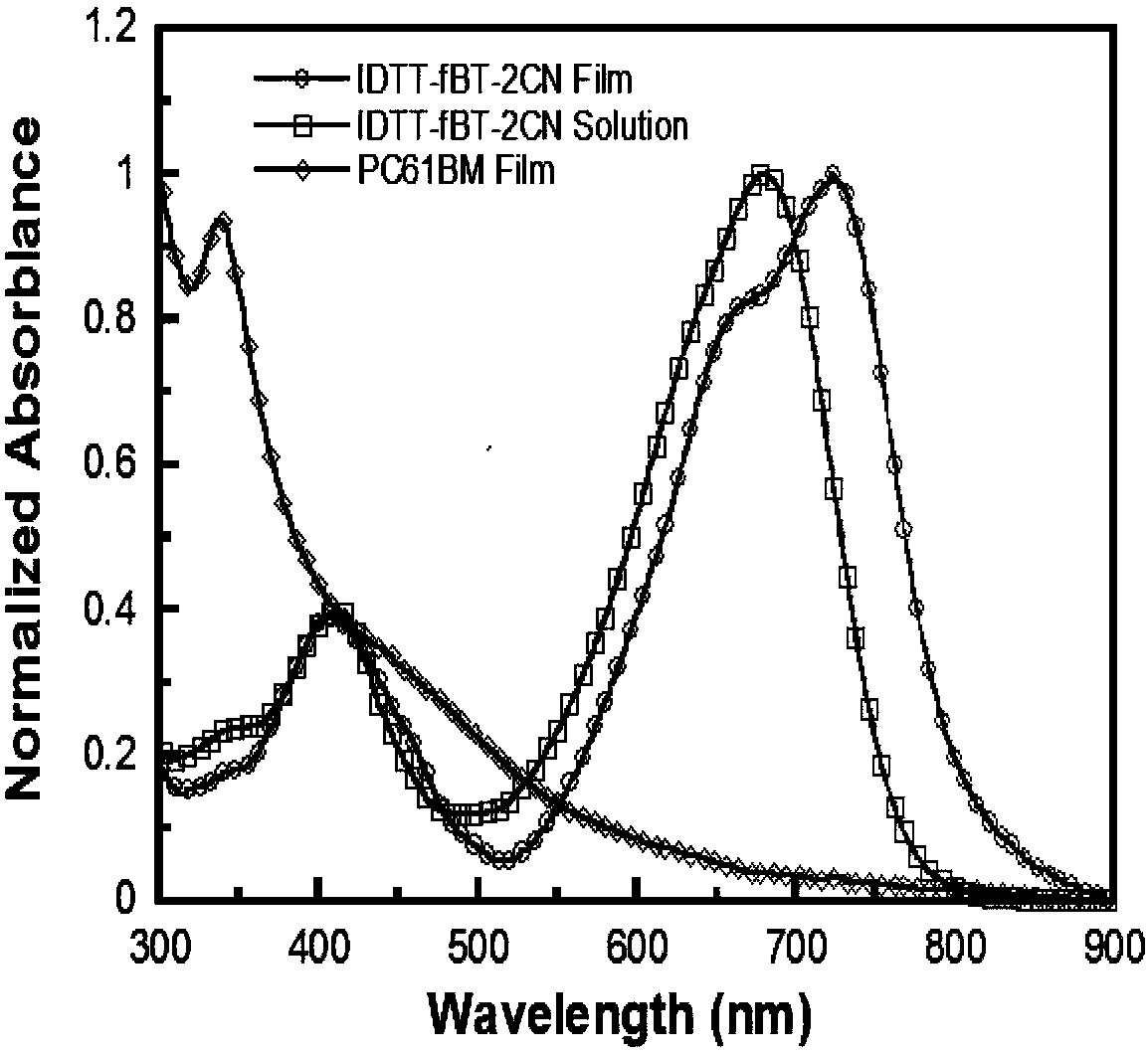
[0024] In the present invention, a fluorine atom is connected to the small molecule of benzothiadiazole to improve the overall electronegativity and electron mobility of the conjugated molecule, and enhance its absorption spectrum, which can be used as an acceptor material to prepare solar cell devices , for higher performance.
[0025] As a preferred version, the 2-((6-fluoro-7-ylbenzo[c][1,2,5]thiadiazole-4-yl)methylene)malononitrile is prepared according to the following method: Using basic alumina as a catalyst, re...
Embodiment 1
[0038] 5-fluoro-3-methyl-1,2-phenylenediamine
[0039] In a 250mL three-necked flask, add the initial raw material 4-fluoro-2methyl-1,6-nitroaniline (5g, 29.4mmol), add reduced iron powder (8.2g, 147mmol), and ethanol (50mL) as a solvent , after the addition is complete, perform pumping and ventilation, make the reaction under a nitrogen atmosphere, and then heat to reflux at 80°C. After the temperature reaches the set value, dilute 30.6mL hydrochloric acid (12molL-1) and 20mL ethanol solvent, and then slowly Drop into the reaction bottle, after the drop is completed, continue to heat the reaction under reflux for more than 5 hours, HCl will volatilize during the reaction, and treat the tail gas with aqueous sodium carbonate solution. After the reaction was completed, the reaction solution was poured into aqueous sodium hydroxide solution, stirred for 5 minutes, added ethyl acetate for extraction, allowed to stand for layers, poured out the upper liquid, then added anhydrous m...
Embodiment 2
[0055] Manufacturing process of polymer solar cell devices:
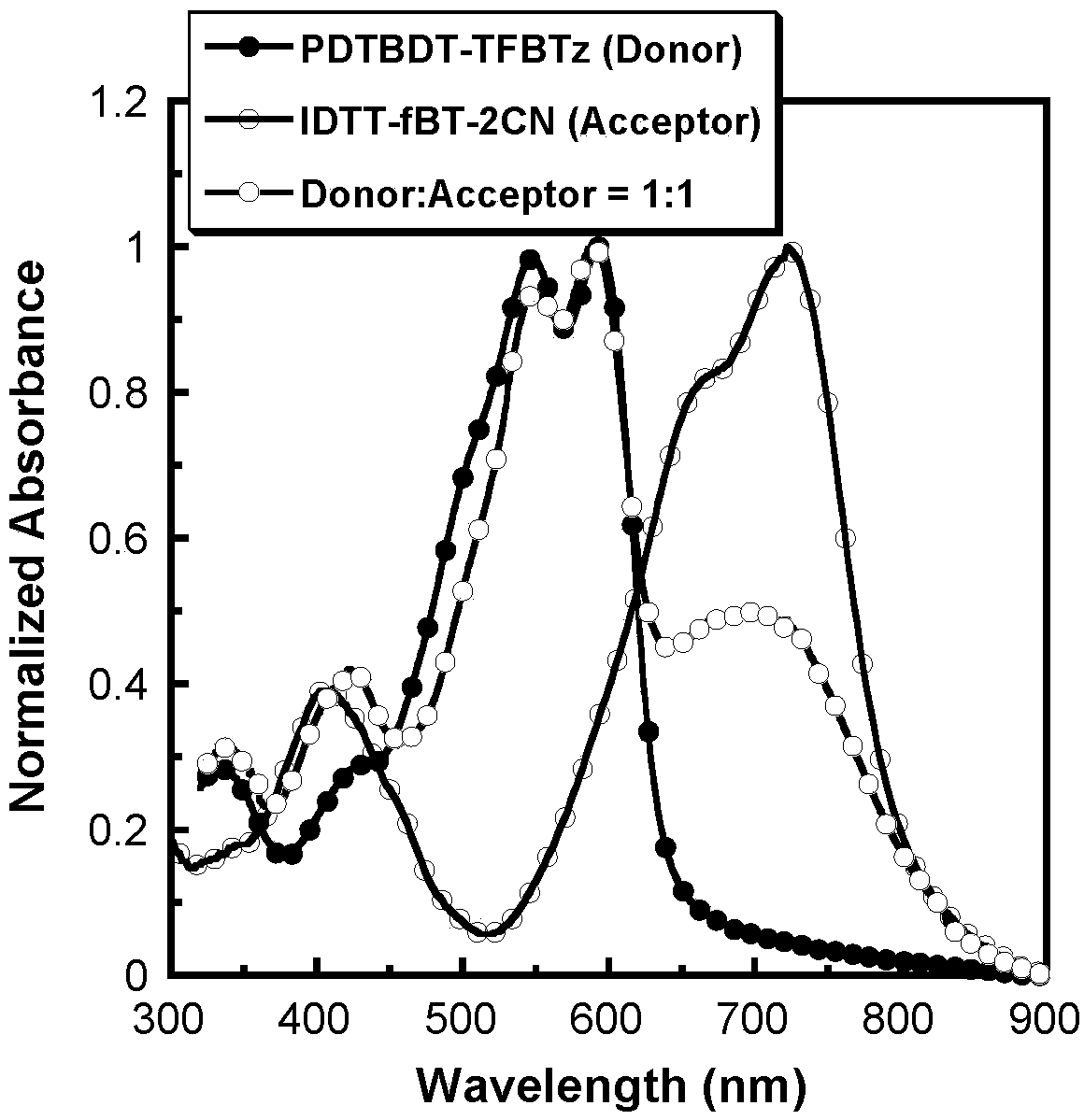
[0056]The device was fabricated by combining with the donor material DTBDT-TFBTz (structure as follows),
[0057]
[0058] The structure of the polymer solar cell device is ITO / PEDOT:PSS / active layer / PFN / Al. First, a 40nm-thick anode modification layer PEDOT:PSS was spin-coated on the ITO substrate after plasma treatment for 3 minutes, and annealed at 150°C for 20 minutes. Then, the acceptor material IDTT-fBT-2CN and the donor material DTBDT-TFBTz were blended (mixing ratio 1:1, wt / wt), using chloroform as a solvent, and then spin-coated on PEDOT:PSS as a photoactive layer, The thickness is about 80nm, and it is divided into 5 groups of samples, namely no annealing treatment, 80°C, 100°C, 120°C, 150°C annealing treatment, and the annealing time is 10min. PEDOT:PSS and active layer thickness were tested using Tencor Alpha-step 500 surface profiler. The cathode modification layer PFN was dissolved in methanol (0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com