Isoindigo-based narrow band gap donor material and preparation method thereof
A narrow band gap and donor technology, applied in the field of organic solar cell materials, can solve the problems of low solubility of poly-3-hexylthiophene, unfavorable processing of organic solar cells, etc., and achieve excellent short-circuit current and good solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
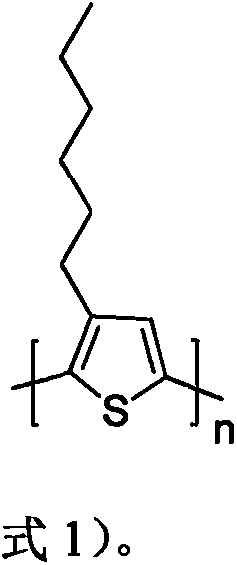
[0030] Correspondingly, the present invention also provides a preparation method of a narrow bandgap donor material based on isoindigo, comprising the following steps: step a1) under the protection of nitrogen, the first compound shown in formula 3, the second compound shown in formula 4 Mix the two compounds, tetrakis(triphenylphosphine)palladium and toluene, heat to 80-120°C and stir for 20-30 hours, cool,
[0031]
[0032] Step a2) filtering and drying the organic phase obtained in step a1 to obtain a narrow band gap donor material.
[0033] As a preferred version, the molar ratio of the first compound, the second compound and tetrakis (triphenylphosphine) palladium is preferably 85-90:40-45:4-6, more preferably 86-90:40-44: 5~6. In step a1, the heating temperature is preferably 100°C, and the stirring time is preferably 24 hours.
[0034] As a preferred solution, the step a2 is: filter the organic phase obtained in the step a1 through diatomaceous earth, dry the filtr...
Embodiment 1
[0056] Embodiment 1: Preparation of narrow bandgap donor material based on isoindigo
[0057] Step 1): Preparation of the third compound
[0058] To the reaction vessel was added 5-hexyl-2,2-dithiophene (1.17 g, 4.7 mmol), followed by 20 mL of anhydrous diethyl ether. The reaction system was placed in liquid nitrogen and cooled to -78°C. At -78°C, n-butyl lithium in n-hexane (2.5 mol / L, 2.8 mL, 6.9 mmol) was added to the reaction system. Then, the reaction system was stirred at -78°C for 1 hour. Add 5 mL of a diethyl ether solution of tributyltin chloride (1.4 g, 6.9 mmol) into the reaction system. Subsequently, the reaction system was stirred at room temperature for 12 hours. After the reaction, the organic phase was repeatedly washed with deionized water, dried over anhydrous magnesium sulfate, and the solvent was removed by vacuum rotary evaporation to obtain the third compound, a total of 1.9 g, with a yield of 99%.
[0059] 1 H NMR (CDCl 3 , 300MHz, 298K) δ7.22(d, ...
Embodiment 2
[0075] Example 2: Preparation of organic solar cells and device performance measurement
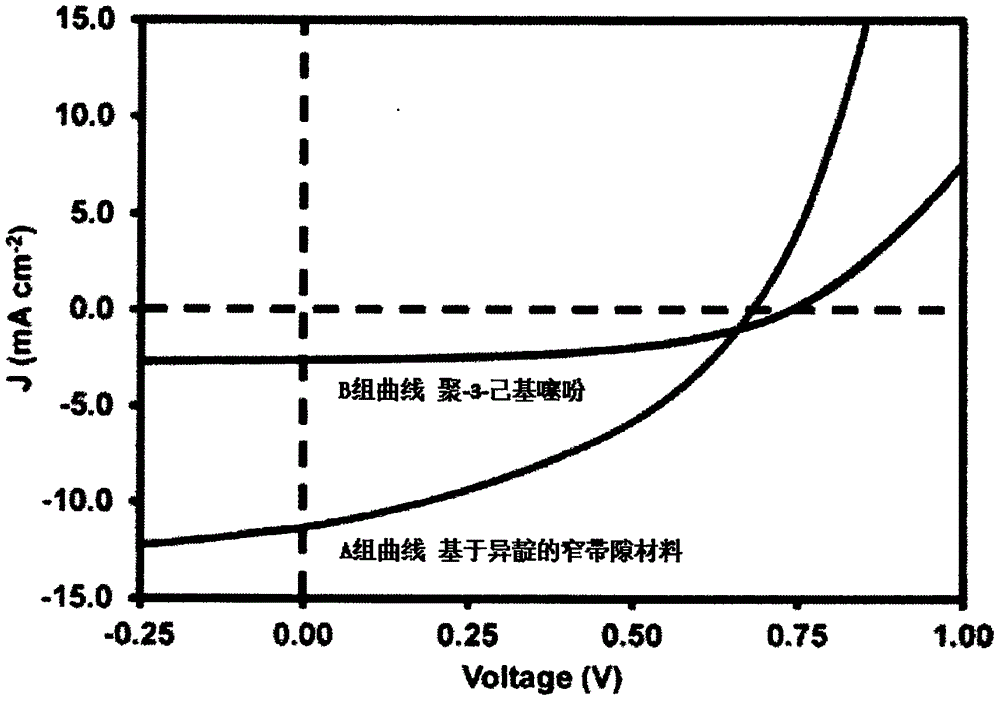
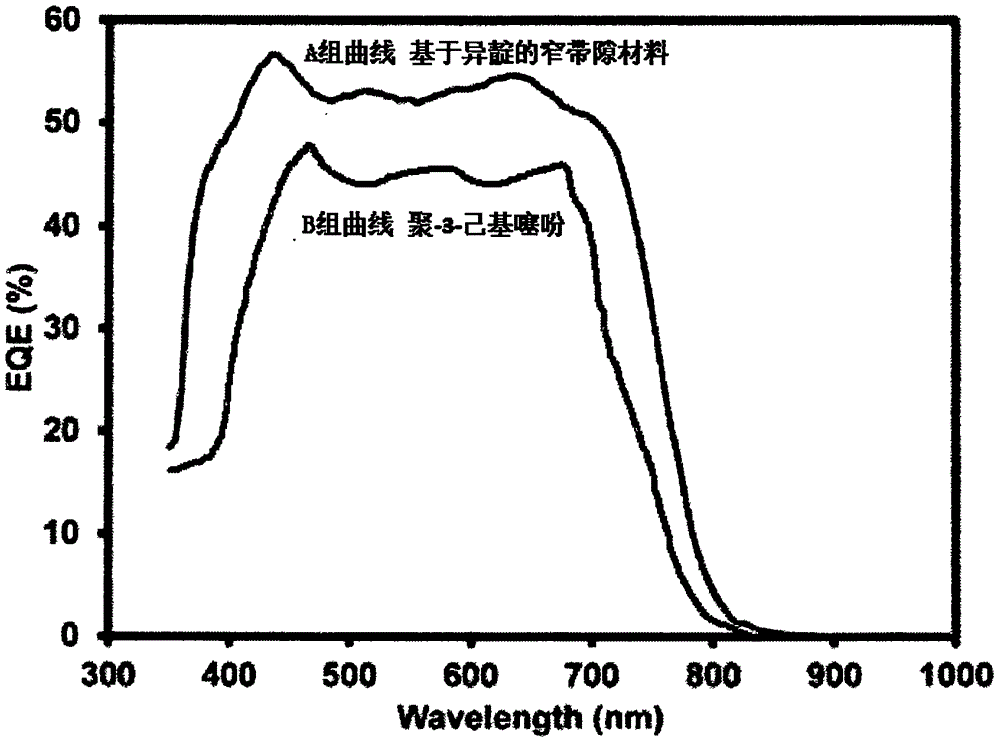
[0076] Poly-3-hexylthiophene is a commonly used donor material in organic solar cells, and it is used as a reference material in device performance testing. The isoindigo-based narrow bandgap material and poly-3-hexylthiophene prepared in Example 1 are respectively used as donor materials, and [6,6]-phenyl-carbon 61-butyric acid methyl ester (PC61BM) is used as acceptor materials, Group A and Group B organic solar cells were prepared.
[0077] Place an ITO (indium tin oxide) conductive glass sheet in a glove box, and spin-coat an active layer film with a thickness of 100 nm. In the active layer, the blending mass ratio of the donor material and PC61BM is 1:1, and chloroform is selected as the solvent. The ITO glass sheet with the active layer spin-coated was transferred to an evaporation chamber, and a layer of Al with a thickness of 60 nm was evaporated to prepare an organic solar cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com