Aryl-substituted terpyridyl compounds, and preparation method and application thereof
A terpyridine and compound technology, which is applied in chemical instruments and methods, organic chemistry, semiconductor/solid-state device manufacturing, etc. The effect of obtaining, simple reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of 2-bromophenyl-2,2':6'2"-terpyridine (II)
[0042]
[0043] In a round bottom flask containing 200 mL of methanol, 4-bromobenzaldehyde (2.0 g, 10.80 mmol), 2-acetylpyridine (4.4 g, 21.6 mmol), sodium hydroxide (0.44 g, 10.80 mmol) and 60mL of concentrated ammonia water (28%) was heated to 70°C and refluxed for 72h. After cooling to room temperature, a gray precipitate was obtained, which was filtered, and the filter cake was washed with water and methanol successively, and dried to obtain 2-bromophenyl-2,2':6'2"-terpyridine (3.8g, 89%). 1 H NMR (CDCl 3, 400MHz): δppm7.36-7.40(m, 3H), 7.48(t, J=8.0Hz, 2H), 7.67(d, J=8.0Hz, 2H), 7.74(d, J=8.0Hz, 2H) , 7.87-7.91(m, 2H), 8.00(d, J=8.0Hz, 2H), 8.68(d, J=8.0Hz, 2H), 8.73-8.76(m, 2H), 8.80(s, 2H).
Embodiment 2
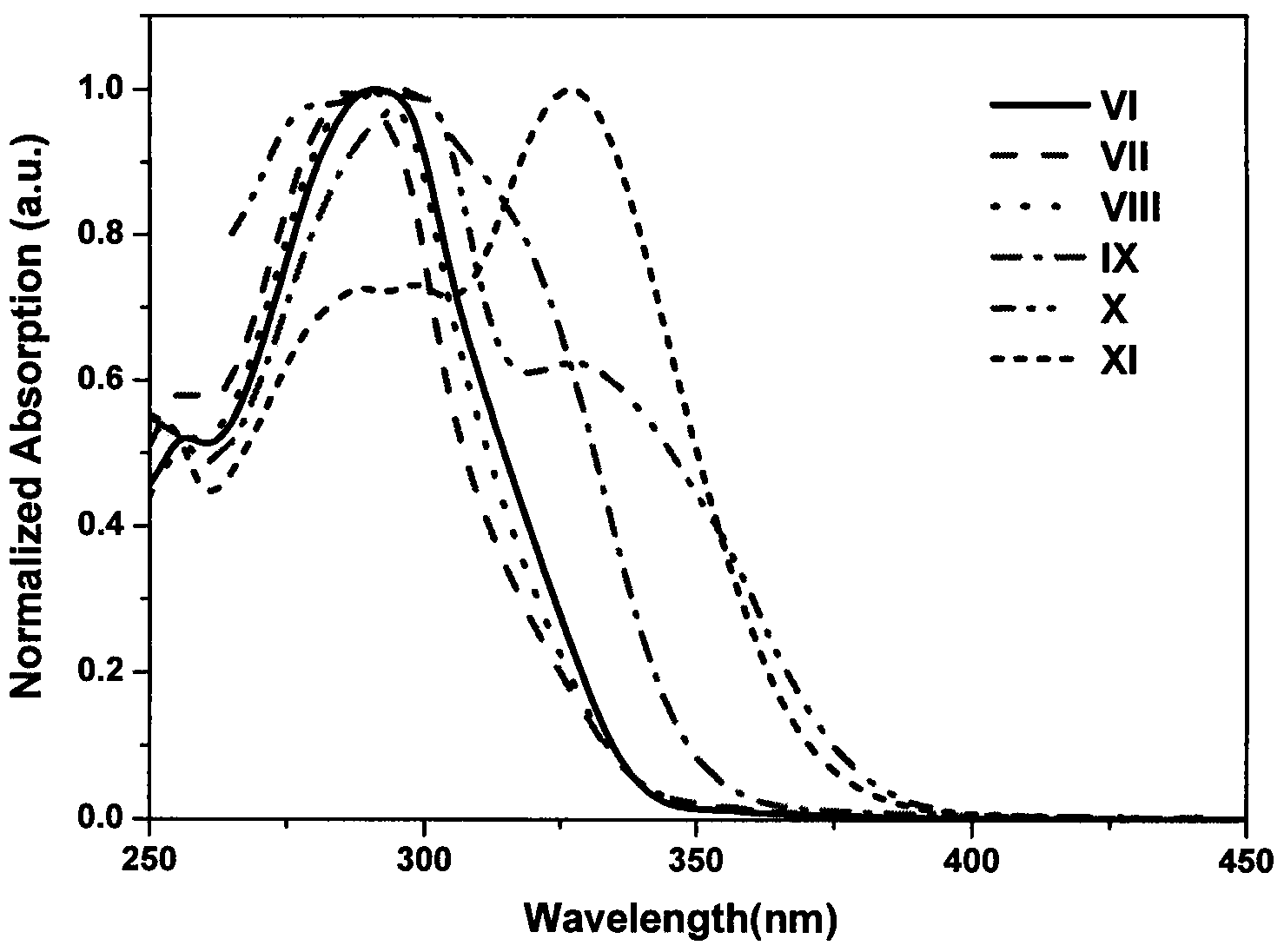
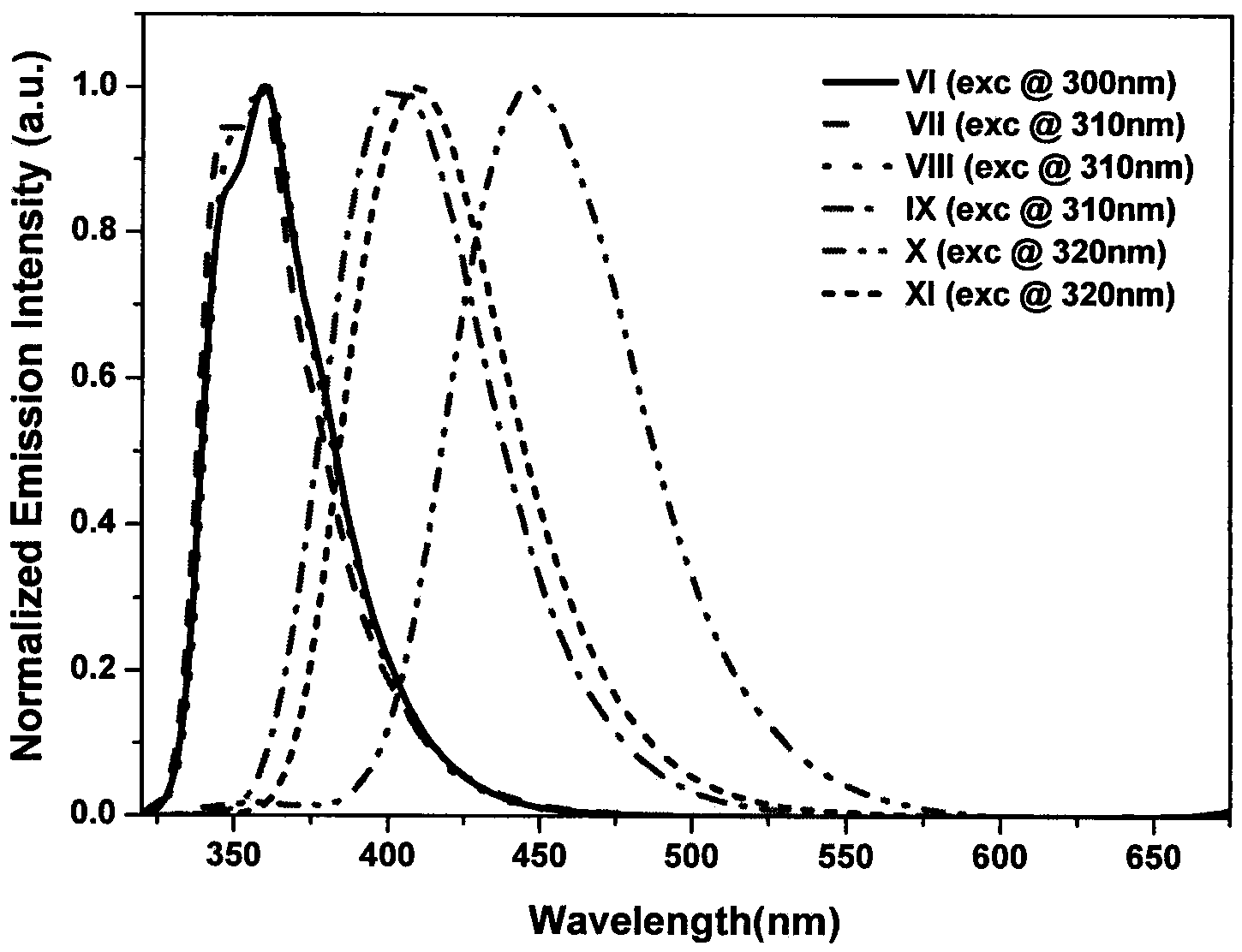
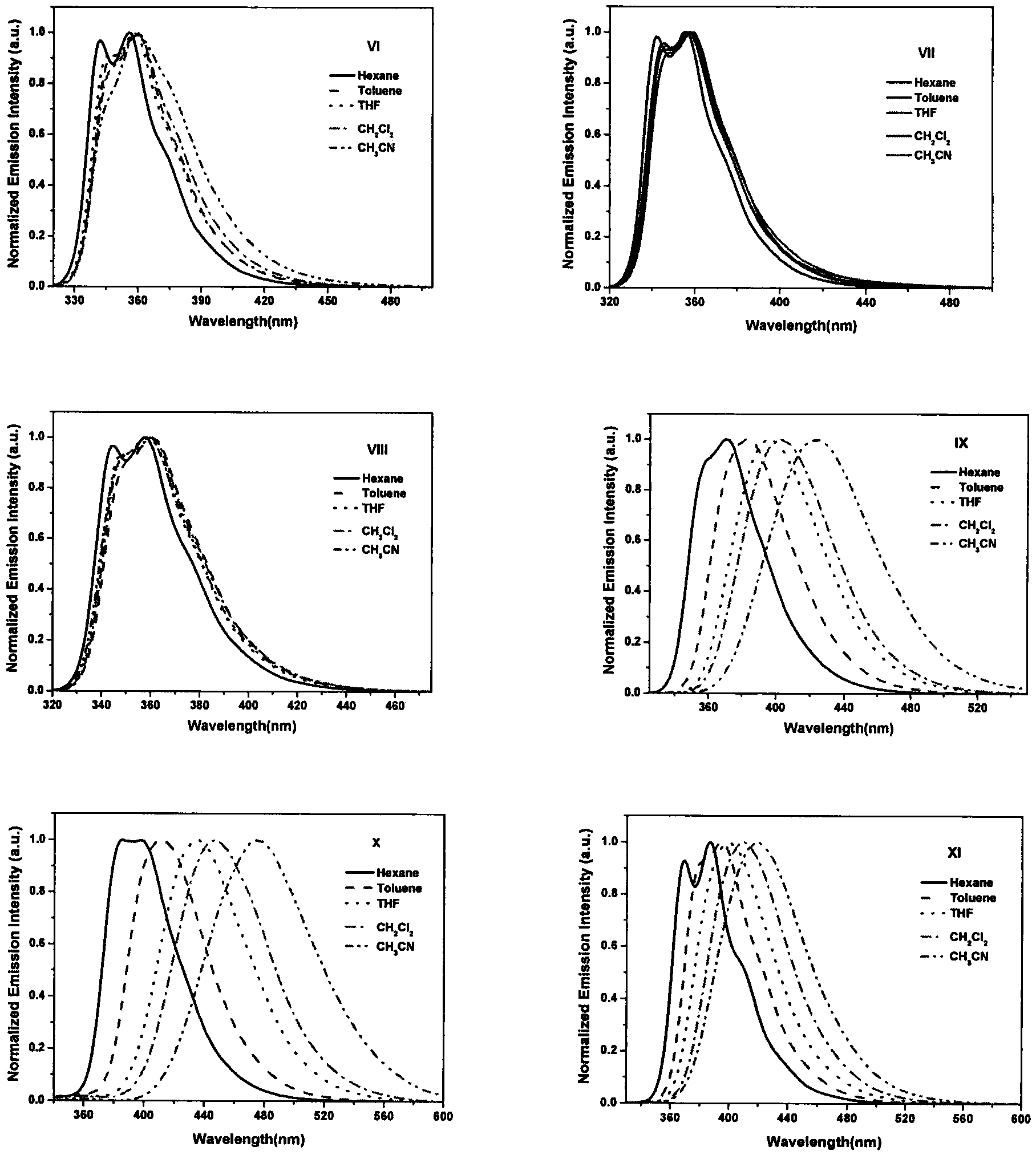
[0044] Embodiment 2: the synthesis of compound shown in formula VI:
[0045]
[0046] Under nitrogen protection, II (388mg, 1mmol), potassium carbonate (552mg, 4.0mmol), tetrakis(triphenylphosphine) palladium (46mg, 0.04mmol), naphthalene borate (1.75g, 7.0mmol) were all added Into the Shrek tube, vacuum and replace with nitrogen three times. in N 2 Under a protective atmosphere, take 10 mL of ultrasonically degassed toluene: water = 4: 1 mixed solvent, inject it into a Shrek tube, then heat to reflux, and react for more than 20 h. TLC tracking shows that no raw material remains, and the reaction ends. Turn off the heating device, wait for the reaction system to cool down to room temperature, dilute with 20 mL of dichloromethane, wash with saturated brine three times, filter out insoluble matter, and collect the organic phase. After drying with anhydrous magnesium sulfate, the solvent was removed under reduced pressure to obtain a crude product. Using a mixed solvent of...
Embodiment 3
[0048] Embodiment 3: the synthesis of compound shown in formula VII:
[0049]
[0050] Under nitrogen protection, Compound VII was synthesized according to the method in Example 2. The product was a white solid with a yield of 85.0% and a melting point of 229-231°C. 1 H NMR (CDCl 3 , 400MHz): δppm7.01(d, J=2.0Hz, 2H), 7.34-7.38(m, 2H), 7.62(dd, J=2.0Hz, 2H), 7.7(d, J=8.0Hz, 2H) , 7.85-7.90(m, 2H), 7.95-8.03(m, 4H), 8.69(d, J=8.0Hz, 2H), 8.73-8.77(m, 3H), 8.80(s, 2H).TOF-MS (ES + )calcd.for C 28 h 21 N 3 O[M+1] + :462.2, found462.1.
[0051] The fluorescence lifetime of this product is 2.15ns, and the test method is the same as that in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com