Novel polyester chain extender and preparation method thereof
A technology of chain extender and polyester is applied in the field of novel polyester chain extender and its preparation, which can solve the problems of increasing side reactions in the reaction system, affecting the performance of the final product, and increasing the preparation cost, so as to reduce the content of terminal carboxyl groups and improve the Use value, high molecular weight effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
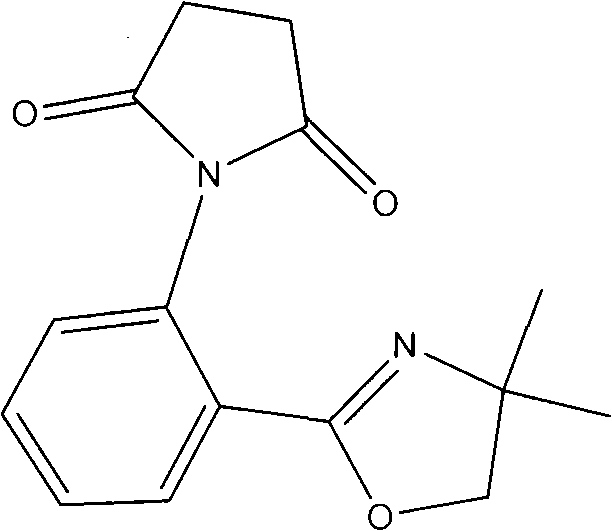
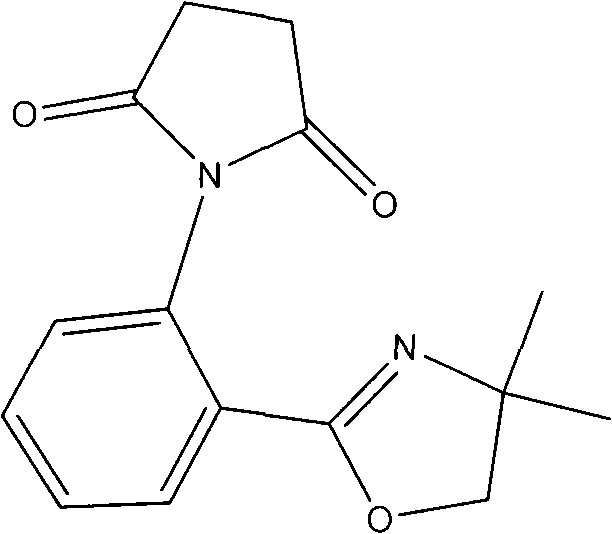
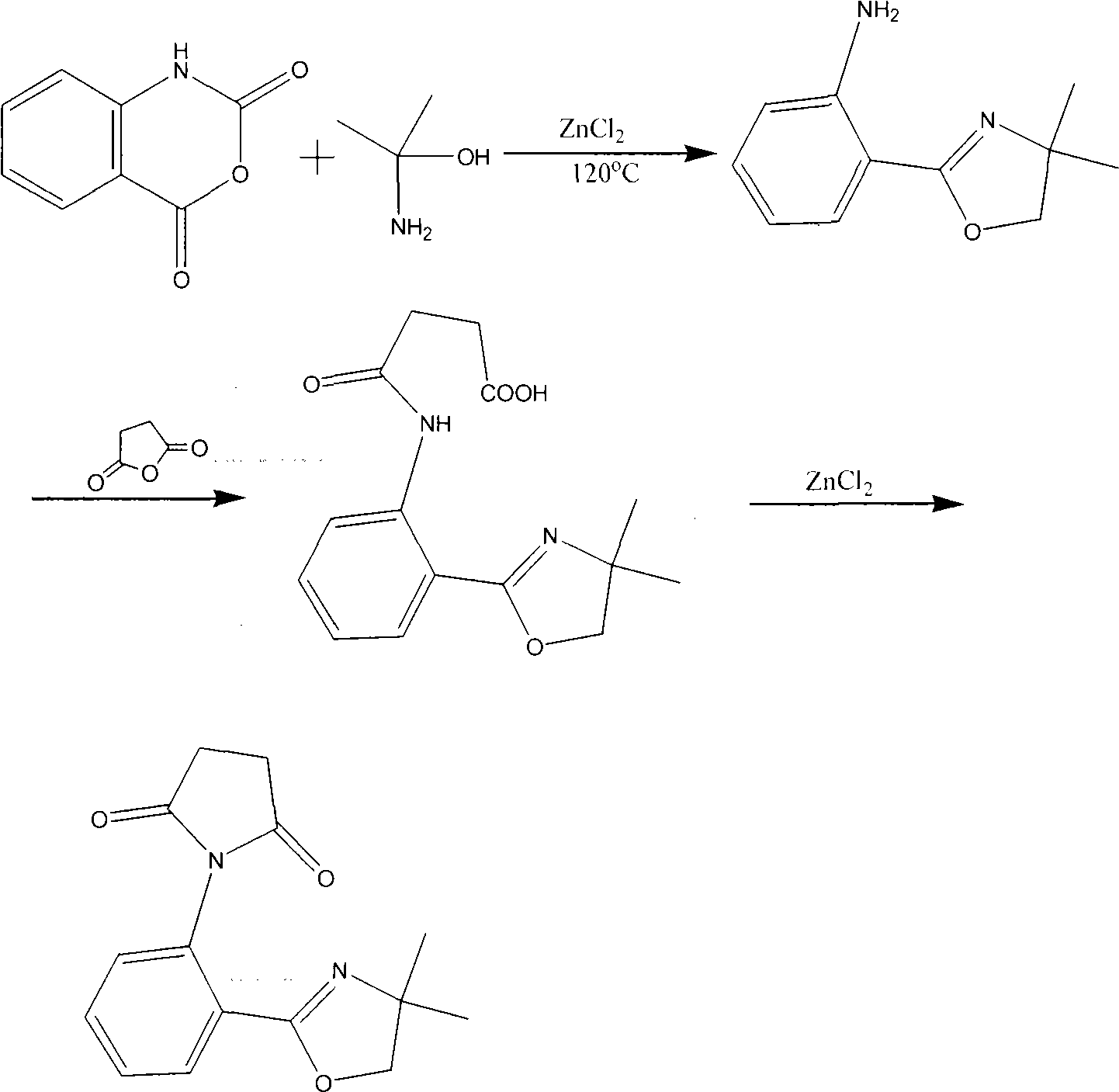
[0018] 1. The molar ratio is 1: 2 isatoic anhydride and 2-aminoalcohol are dissolved in the ratio of 20g / L in chlorobenzene, add the ZnCl of 0.08 of the total mass of isatoic anhydride and 2-aminoalcohol 2 As a catalyst, react at 100°C for 20 h, remove the chlorobenzene solvent from the reaction solution to obtain a crude product, and purify by recrystallization to obtain oxazoline aniline.
[0019] 2. Dissolve the obtained oxazoline aniline in ether at a ratio of 45 g / L. After the solid is completely dissolved, add succinic anhydride. The molar ratio of succinic anhydride to oxazoline aniline is 1:0.75, and stir at room temperature for 2 h , to obtain a suspension. The reaction solution was filtered to obtain the intermediate, which was used in the next step of synthesis.
[0020] 3. Mix the intermediate with a mass ratio of 1.0:0.3:6.0, anhydrous zinc chloride and diatomaceous earth, and grind them into fine powder. Under 380W microwave irradiation power, heat for 5min. A...
Embodiment 2
[0022] 1. be that 1: 2.4 isatoic anhydride and 2-aminoalcohol are dissolved in chlorobenzene in the ratio of 20g / L by molar ratio, add the ZnCl of 0.14% of described isatoic anhydride and 2-aminoalcohol gross mass 2 As a catalyst, it was reacted at 120°C for 23 hours, and the reaction solution was removed from the chlorobenzene solvent to obtain a crude product, which was purified by recrystallization to obtain oxazoline aniline.
[0023] 2. Dissolve the obtained oxazoline aniline in ether at a ratio of 45 g / L. After the solid is completely dissolved, add succinic anhydride. The molar ratio of succinic anhydride to oxazoline aniline is 1:0.82, and stir at room temperature for 4 h , to obtain a suspension. The reaction solution was filtered to obtain the intermediate, which was used in the next step of synthesis.
[0024] 3. Mix the intermediate with a mass ratio of 1.0:0.8:7.0, anhydrous zinc chloride and diatomaceous earth, and grind them into fine powder. Under 500W microw...
Embodiment 3
[0026] 4. be that 1: 2.8 isatoic anhydride and 2-aminoalcohol are dissolved in chlorobenzene in the ratio of 20g / L by molar ratio, add the ZnCl of 0.2% of described isatoic anhydride and 2-aminoalcohol gross mass 2 As a catalyst, react at 120°C for 26 hours, remove the chlorobenzene solvent from the reaction solution to obtain a crude product, and purify by recrystallization to obtain oxazoline aniline.
[0027] 5. Dissolve the obtained oxazoline aniline in ether at a ratio of 45 g / L. After the solid is completely dissolved, add succinic anhydride. The molar ratio of succinic anhydride to oxazoline aniline is 1:0.9, and stir at room temperature for 6 h , to obtain a suspension. The reaction solution was filtered to obtain the intermediate, which was used in the next step of synthesis.
[0028] 6. Mix the intermediate with a mass ratio of 1.0:1.2:7.5, anhydrous zinc chloride and diatomaceous earth, and grind them to a fine powder. Under 800W microwave irradiation power, heat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com