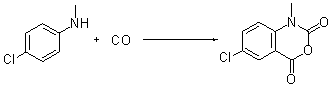
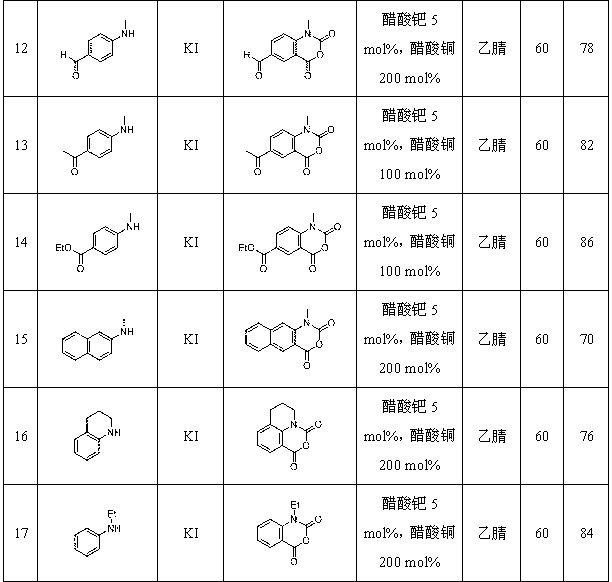
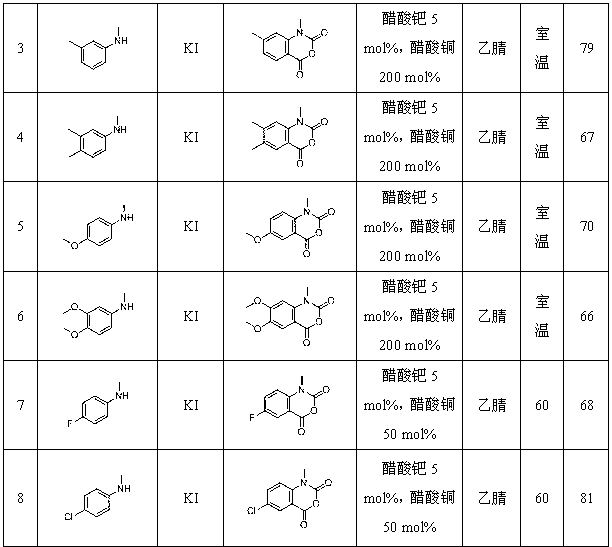
Synthetic method for isatoic anhydride derivative
A technology for the synthesis of isatoic anhydride and its synthesis method, which is applied in the field of synthesis of isatoic anhydride derivatives, can solve the problems of harsh reaction conditions, high cost, and reduced yield, and achieve mild reaction conditions, low production cost, and high synthesis yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: prepare N-methylisatoic anhydride by N-methylaniline
[0027]
[0028] Add N-methylaniline (0.54 g, 0.005 mol), copper acetate (0.36 g, 0.002 mol), potassium iodide (0.17 g, 0.001 mol), palladium acetate (0.056 g, 0.00025 mol, 5 mol) into a 100 mL round bottom flask %) and 50 mL of acetonitrile, heated to 60°C in a mixture of carbon monoxide and oxygen at a ratio of 5:1 at an atmospheric pressure, detected the reaction by thin-layer chromatography, and cooled to room temperature after 11 hours, and the reaction mixture was decomposed with a short silica gel column The inorganic salt in the reaction was filtered off, the filtrate was spin-dried in vacuum, and the crude product of the reactant was recrystallized or separated by column chromatography to obtain 0.60 g of the pure product, which was a yellow solid with a yield of 68%.
[0029] Structural analysis: 1 H NMR (DMSO- d 6 , 400 MHz): δ = 7.97 (d, J = 6.8 Hz, 1 H), 7.85-7.82 (t, J = 6.8 Hz, ...
Embodiment 2
[0030] Embodiment 2: Similar to Embodiment 1, the difference is that potassium iodide is not added, and the reaction yield is only 40%.
Embodiment 3
[0031] Example 3: Preparation of 3,4-dimethyl-N-methylisatoic anhydride from 3,4-dimethyl-N-methylaniline
[0032]
[0033] Add 3,4-dimethyl-N-methylaniline (27.0 mg, 0.2 mmol), copper acetate (80.0 mg, 0.44 mol), potassium iodide (6.6 mg, 0.04 mmol) and palladium acetate ( 2.2 mg, 0..01 mmol) and 2 mL of acetonitrile, reacted at room temperature in carbon monoxide at an atmospheric pressure, detected the reaction by thin-layer chromatography until the reaction was completed, extracted the reaction mixture with water and ethyl acetate, combined the organic phases and vacuumed After spinning to dryness, the crude product of the reactant was separated by column chromatography to obtain 27.5 mg of the pure product, the product was a yellow solid, and the yield was 67%.
[0034] Structural analysis: 1 H NMR (DMSO- d 6 , 400 MHz): δ = 7.66 (s, 1 H), 7.20 (s, 1 H), 3.39 (s, 3 H), 2.33 (s, 3 H), 2.22 (s, 3 H); 13 C NMR (DMSO- d 6 , 100 MHz): δ = 158.8, 147.9, 147.7, 140.3, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com