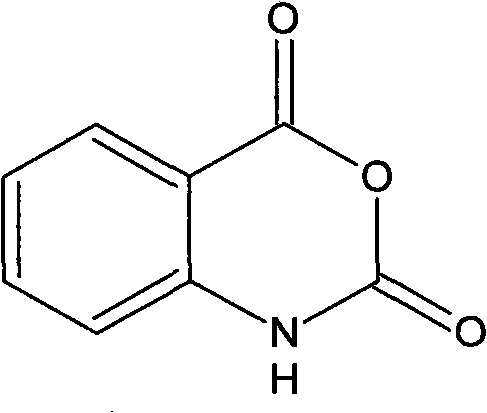
Method for synthesizing isatoic anhydride
A technology of isatoic anhydride and phthalimide, applied in the direction of organic chemistry, can solve the problems of reduced yield, difficult treatment, difficult waste water, etc., and achieve the effect of mild reaction conditions and reduced generation and emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
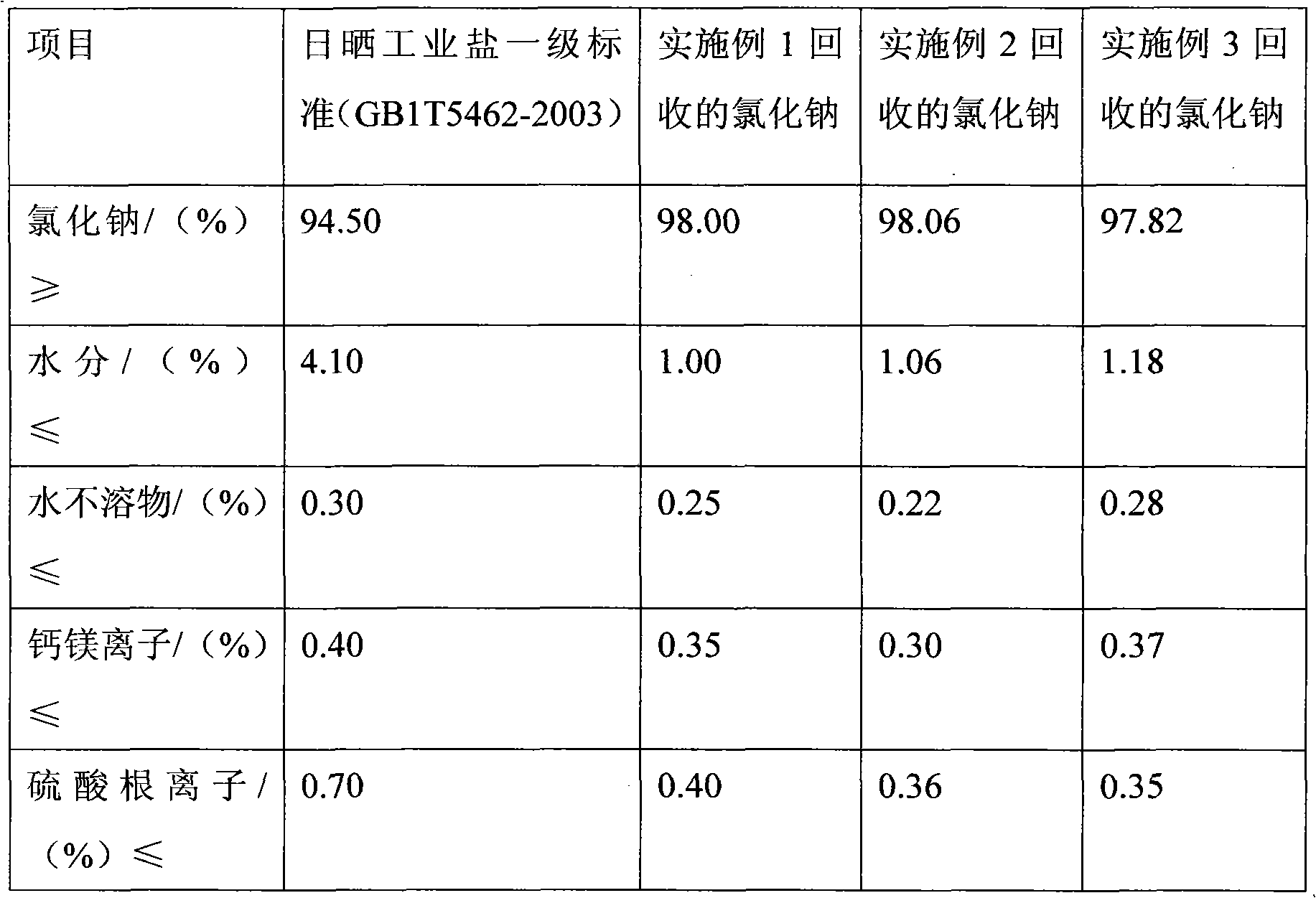
[0021] Add 150 milliliters of ethylene dichloride to a 250 milliliter four-neck flask equipped with a stirrer, a temperature device, a reflux device, a chlorine passage device and a hydrogen chloride tail gas absorption device. After starting the stirring, add 25.0 grams of phthalimide (content 99%, 0.168mol), heat to 60-65°C and stir to dissolve; cool down to 40-45°C, and then pass 12.1 grams of chlorine gas (content 99%, 0.169 mol), stirred for 1.5 hours, slowly added 6.9 grams of solid alkali sodium hydroxide (content 98%, 0.169mol) into the bottle and controlled the temperature at 40-45°C, continued to stir for 1 hour after adding, filtered the sodium chloride while it was hot Salt, the filtrate is isatoic anhydride solution. The solution was cooled to 15-20° C., isatoic anhydride was precipitated in the solution, and solid isatoic anhydride was filtered to obtain 25.6 grams of solid isatoic anhydride after drying. The measured content was 99.2%, and the calculated isatoic...
Embodiment 2
[0023] Add 120 milliliters of chloroform in a 250 milliliter four-neck flask equipped with a stirrer, a temperature device, a reflux device, a chlorine passage device and a hydrogen chloride tail gas absorption device. After starting the stirring, add 25.0 grams of phthalimide (content 99%, 0.168mol), heat to reflux state, stir and dissolve; cool down to 40-45°C, and then pass 12.6 grams of chlorine gas (content 99%, 0.176 mol), stirred for 2 hours, slowly added 7.2 grams of solid alkali sodium hydroxide (content 98%, 0.176mol) into the bottle and controlled the temperature below 45°C, continued to stir for 1 hour after adding, filtered the sodium chloride salt while it was hot , The filtrate is isatoic anhydride solution. Cool the solution to 15-20°C to precipitate isatoic anhydride, filter and dry 25.5 grams, and the measured content is 99.0%. The product yield of isatoic anhydride calculated by analysis is 92.4%. The sodium chloride recovered by filtration can meet the fi...
Embodiment 3
[0025] Add 150 milliliters of dichloromethane to a 250 milliliter four-neck flask equipped with a stirrer, a temperature device, a reflux device, a chlorine passage device and a hydrogen chloride tail gas absorption device. After starting the stirring, add 25.0 grams of phthalimide (content 99%, 0.168mol), heat to 60-65°C and stir to dissolve; cool down to 40-45°C, and then pass 12.1 grams of chlorine gas (content 99%, 0.169 mol), stirred for 2 hours, slowly added 7 grams of solid alkali sodium hydroxide (content 98%, 0.172mol) into the bottle and controlled the temperature at 40-45°C, continued to stir for 1 hour after adding, filtered the sodium chloride while it was hot Salt, the filtrate is isatoic anhydride solution. The solution was cooled to 15-20° C., isatoic anhydride was precipitated in the solution, and solid isatoic anhydride was obtained by filtration, 25.4 grams after drying, the measured content was 99.1%, and the calculated isatoic anhydride product yield was 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com