Tryptanthrin derivative and medicinal use thereof
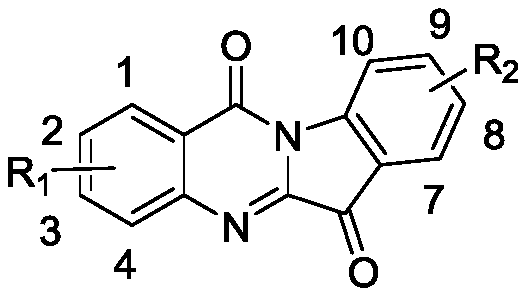
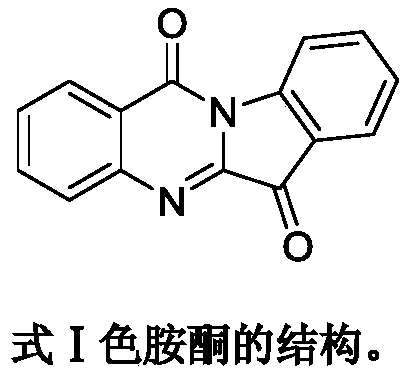
The technology of a derivative, tryptanthrin, is applied in the field of new tryptanthrin derivatives and their medicinal uses, which can solve the problems of difficult to obtain and isatoic anhydride derivatives, and achieve the goal of inhibiting tumor cells and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
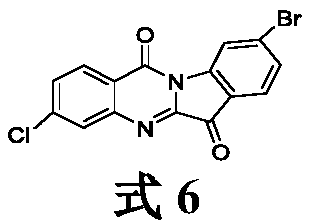
[0071] Example 1. Synthesis of 2,7-dichloroindo[2,1-b]quinazoline-6,12-dione (1a):
[0072] Weigh 1.0g (0.0055mol) of 4-chloroisatin and 1.15g (0.0058mol) of 5-chloroisatic anhydride into a 100ml three-necked bottle equipped with a magnet, then add 40ml of chloroform to the three-necked bottle , 1.5ml of triethylamine, heating and refluxing in an oil bath at 80°C for 3h, after the reaction, stop heating, wait for cooling, and suction filter to obtain a yellow solid powder of the crude product, wash the product with 10ml of ethanol for 3 times, and obtain a yellow solid powder of the pure product , dried and weighed 1.12g, yield 64.4%.
[0073] 2,7-dichloroindole[2,1-b]quinazoline-6,12-dione (1a):
[0074] Yellow solid powder, yield 64.4%; m.p.291.2-293.4°C; 1 H NMR (400MHz, DMSO) δ8.47 (d, J = 8.1Hz, 1H), 8.29 (dd, J = 1.8, 1.0Hz, 1H), 8.02 (s, 1H), 7.92–7.84 (m, 1H) ,7.56(t,J=7.7Hz,1H),7.16(s,1H);IR(KBr)ν:3081,3030,1733,1686,1584,1549,1460,1418,915,796,726cm -1 ; EI-MS: c...
Embodiment 2
[0075] Example 2. Synthesis of 2,9-dichloroindo[2,1-b]quinazoline-6,12-dione (1b):
[0076] Weigh 0.3g (0.0017mol) of 6-chloroisatin and 0.36g (0.0018mol) of 5-chloroisatic anhydride and add them to a 100ml three-necked bottle with a magnet, then add 40ml of chloroform to the three-necked bottle , 1.5ml of triethylamine, heating and refluxing in an oil bath at 80°C for 3h, after the reaction, stop heating, wait for cooling, and suction filter to obtain a yellow solid powder of the crude product, wash the product with 10ml of ethanol for 3 times, and obtain a yellow solid powder of the pure product , dried, weighed 0.34g, and the yield was 63.0%.
[0077] 2,9-Dichloroindole[2,1-b]quinazoline-6,12-dione (1b):
[0078] Yellow solid powder, yield 63.0%; m.p.274.5-276.4°C; 1 H NMR (400MHz, DMSO) δ8.02(s, 1H), 7.94(s, 1H), 7.54(d, J=8.1Hz, 1H), 7.13(d, J=1.8Hz, 1H), 7.11(d ,J=1.8Hz,1H),6.95(d,J=1.7Hz,1H); IR(KBr)ν:3117,3060,1725,1678,1591,1553,1464,1428,847,775cm -1 ; EI-MS: cal...
Embodiment 3
[0079] Example 3. Synthesis of 2-chloro-9-bromoindo[2,1-b]quinazoline-6,12-dione (1c):
[0080] Weigh 0.70g (0.0031mol) of 6-bromoisatin and 0.66g (0.0033mol) of 5-chloroisatic anhydride and add them to a 100ml three-necked bottle equipped with a magnet, then add 40ml chloroform to the three-necked bottle , 1.5ml triethylamine, heating and refluxing in an oil bath at 80°C for 3h, after the reaction, stop heating, wait for cooling, and suction filter to obtain the crude product yellow solid powder, wash the product 3 times with 10ml ethanol to obtain the pure product yellow solid powder , dried, weighed 0.63g, and the yield was 56.3%.
[0081] 2-Chloro-9-bromoindole[2,1-b]quinazoline-6,12-dione (1c):
[0082] Yellow solid powder, yield 56.3%; m.p.283.4-285.1°C; 1 H NMR (400MHz, DMSO) δ8.66(d, J=1.6Hz, 1H), 8.32(d, J=2.1Hz, 1H), 8.05(s, 1H), 7.90(s, 1H), 7.88(s ,1H),7.78(d,J=1.6Hz,1H);IR(KBr)ν:3115,3068,1725,1678,1586,1552,1463,1421,911,843,755cm -1 ; EI-MS: calcd for C 15 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com