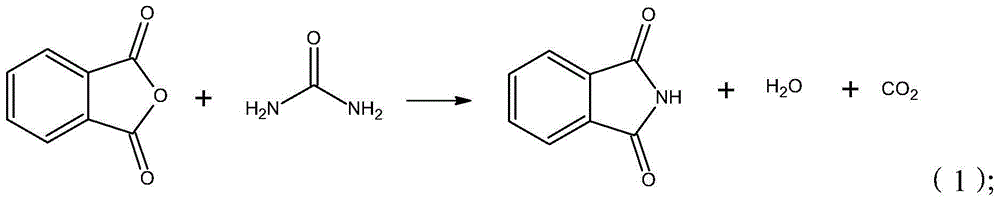
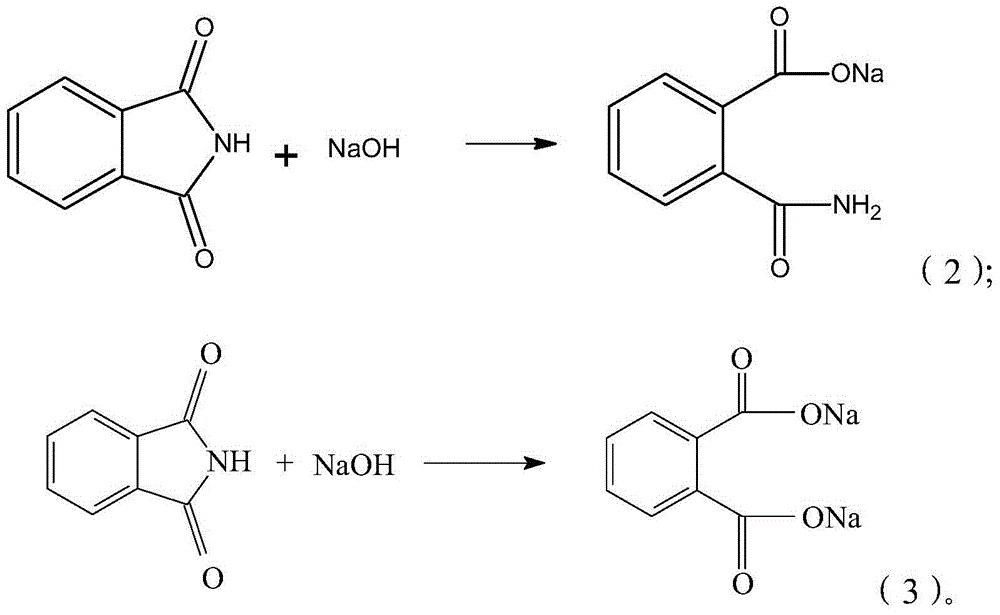
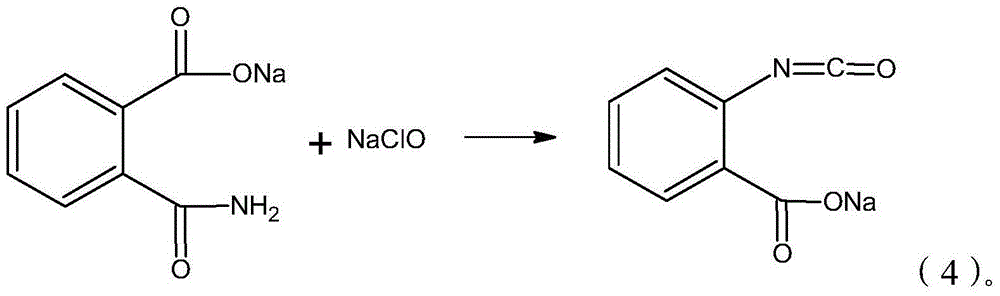
Synthesis process of isatoic anhydride
A synthesis process, the technology of isatoic anhydride, applied in the field of synthesis of isatoic anhydride, can solve the problems of low yield and purity of isatoic anhydride, increase of production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Take 100g of phthalic anhydride and put it in a 2000mL flask, heat and stir to melt for 5-10min;
[0033] 2) Add 21.5g of urea after it is completely melted, and cool down to 10°C after fully reacting;
[0034] 3) Add 500mL of water and 84g of liquid caustic soda with a concentration of 32wt%, continue stirring and dissolving, and cool down to 1°C. When the system becomes clear, the phthalimide is completely dissolved;
[0035] 4) Add sodium hypochlorite solution in the flask, the system changes from clarification to turbidity and becomes clear again, and KI test paper shows obvious color, stops adding sodium hypochlorite, added concentration and is 10wt% sodium hypochlorite solution 424.7g;
[0036] 5) Slowly add 77.2g of hydrochloric acid with a concentration of 31wt% dropwise, adjust the pH=6.4, and crystals are precipitated;
[0037] 6) Suction filtration, and the obtained crystals are dried in an oven.
Embodiment 2
[0039] 1) Take 100g of phthalic anhydride and put it in a 2000mL flask, heat and stir to melt for 5-10min;
[0040] 2) Add 21.5g of urea after it is completely melted, and cool down to 15°C after fully reacting;
[0041] 3) Add 500mL of water and continue stirring to dissolve and cool down. When the temperature drops to about 10°C, add 85g of liquid caustic soda with a concentration of 32wt%, and continue to cool down and dissolve to 1°C. When the system becomes clear, the phthalimide is completely dissolved;
[0042]4) Sodium hypochlorite is added in the flask, and the system changes from clarification to turbidity to clarification again, and the KI test paper shows obvious color, stops adding sodium hypochlorite, and adds concentration and is 427.4g of sodium hypochlorite of 13wt%;
[0043] 5) Slowly add 77g of hydrochloric acid with a concentration of 31wt% dropwise, adjust the pH=6.4, and crystals are precipitated;
[0044] 6) Suction filtration, and the obtained crystals...
Embodiment 3
[0046] 1) Take 100g of phthalic anhydride and put it in a 2000mL flask, heat and stir to melt for 5-10min;
[0047] 2) Add 21.5g of urea after it is completely melted, and cool down to 20°C after fully reacting;
[0048] 3) Add 400mL of water and 85g of liquid caustic soda with a concentration of 32wt%, continue to stir and dissolve, and cool down to 1°C. When the system becomes clear, the phthalimide is completely dissolved;
[0049] 4) Sodium hypochlorite is added in the flask, and the system changes from clarification to turbidity to clarification again, and the KI test paper shows obvious color, stops adding sodium hypochlorite, and the addition concentration is 12wt% sodium hypochlorite 439.3g;
[0050] 5) Slowly add 78g of hydrochloric acid with a concentration of 31wt% dropwise, adjust the pH=6.4, and crystals are precipitated;
[0051] 6) Suction filtration, and the obtained crystals are dried in an oven.
[0052] Table 1 The yield data table of embodiment 1~embodime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com