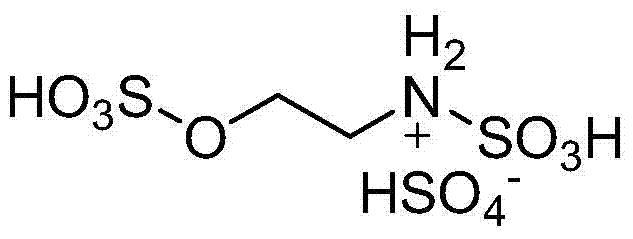
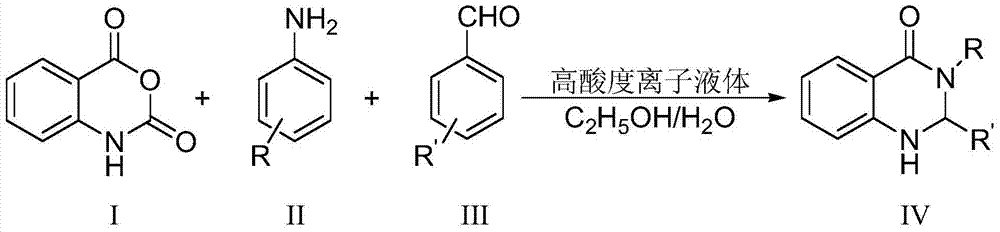
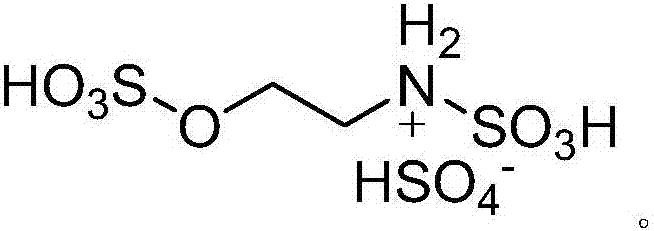
A kind of preparation 2, the method for 3-dihydroquinazoline-4 (1h)-ketone and derivatives thereof
A technology for dihydroquinazoline and derivatives, applied in chemical recovery, organic chemistry, etc., which can solve the problems of complex preparation process, difficult biodegradation, and high preparation cost, and achieve good selectivity, high utilization rate, and low loss Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 5mmol isatoic anhydride, 5mmol aniline, 5mmol benzaldehyde and 0.4mmol high acidity ionic liquid catalyst respectively to 15ml of water and ethanol mixed solution (V (water): V (ethanol) = 1:2) with stirring In a 50ml single-necked bottle, the reflux reaction time was 12min, followed by TLC (thin plate chromatography). After the reaction was completed, a large amount of solids were precipitated after cooling to room temperature. The solids were crushed, left standing, and suction filtered. 2,3-Diphenyl-2,3-dihydroquinazolin-4(1H)-one, yield 94%. The filtrate was directly added with isatoic anhydride, aniline and benzaldehyde for reuse.
[0025] 2,3-diphenyl-2,3-dihydroquinazolin-4(1H)-one: m.p.208~209℃; 1 H NMR (400MHz, DMSO-d 6 ): δ=6.27(d, J=2.4Hz, 1H, CH), 6.68(t, J=7.2Hz, 1H, ArH), 6.75(d, J=8.0Hz, 1H, ArH), 7.16(t, J=7.2Hz, 1H, ArH), 7.24~7.33(m, 8H, ArH), 7.35(d, J=7.2Hz, 2H, ArH), 7.64(d, J=2.4Hz, 1H, NH), 7.70 (d, J=8.0Hz, 1H, ArH)
Embodiment 2
[0027] Add 5mmol isatoic anhydride, 5mmol aniline, 5mmol p-methoxybenzaldehyde and 0.5mmol high acidity ionic liquid catalyst to 15ml water and ethanol mixed solution (V (water): V (ethanol) = 1:2) In a 50ml single-necked bottle with a stirrer, the reflux reaction time is 8min, TLC (thin plate chromatography) tracking detection, after the reaction is completed, a large amount of solids are precipitated when cooled to room temperature, the solids are crushed, left to stand, and suction filtered, and the resulting filter residue is vacuum After drying, pure 2-p-methoxyphenyl-3-phenyl-2,3-dihydroquinazolin-4(1H)-one was obtained in a yield of 90%. The filtrate was directly added with isatoic anhydride, aniline and p-methoxybenzaldehyde and reused.
[0028] 2-p-methoxyphenyl-3-phenyl-2,3-dihydroquinazolin-4(1H)-one: m.p.204~206℃; 1 H NMR (400MHz, DMSO-d 6 ): δ=3.64(s, 3H, OCH 3), 6.21(d, J=2.4Hz, 1H, CH), 6.69(t, J=7.6Hz, 1H, ArH), 6.76(d, J=8.0Hz, 1H, ArH), 6.88(d, J= 8.4Hz, ...
Embodiment 3
[0030] 5mmol isatoic anhydride, 5mmol p-chloroaniline, 5mmol p-tolualdehyde and 0.6mmol high acidity ionic liquid catalyst were respectively added to 25ml water and ethanol mixed solution (V (water): V (ethanol) = 1:2 ) in a 100ml single-necked bottle with a stirrer, the reflux reaction time is 20min, TLC (thin plate chromatography) tracking detection, after the reaction is completed, a large amount of solids are precipitated when cooled to room temperature, the solids are crushed, left standing, and suction filtered, the obtained filter residue Pure 2-p-chlorophenyl-3-p-methylphenyl-2,3-dihydroquinazolin-4(1H)-one was obtained after vacuum drying with a yield of 91%. Repeat use after directly adding isatoic anhydride, p-chloroaniline and p-tolualdehyde in the filtrate.
[0031] 2-p-chlorophenyl-3-p-methylphenyl-2,3-dihydroquinazolin-4(1H)-one: m.p.258~260°C; 1 H NMR (400MHz, DMSO-d 6 ): δ=2.23(s, 3H, CH 3 ), 6.24(d, J=2.4Hz, 1H, CH), 6.77(dd, J=7.6Hz, 2H, ArH), 7.11(s, 4H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com