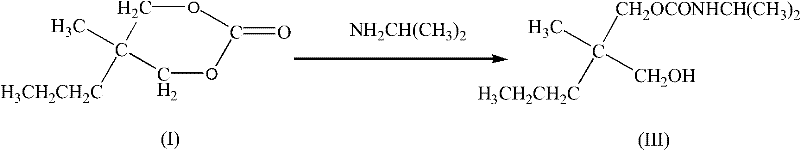Patents
Literature
37 results about "Isopropamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isopropamide (R5) is a long-acting anticholinergic drug. It is used in the treatment of peptic ulcers and other gastrointestinal disorders involving hyperacidity (gastrointestinal acidosis) and hypermotility. Chemically, it contains a quaternary ammonium group. It is most often provided as an iodide salt, but is also available as a bromide or chloride salt. It was discovered at Janssen Pharmaceutica in 1954.
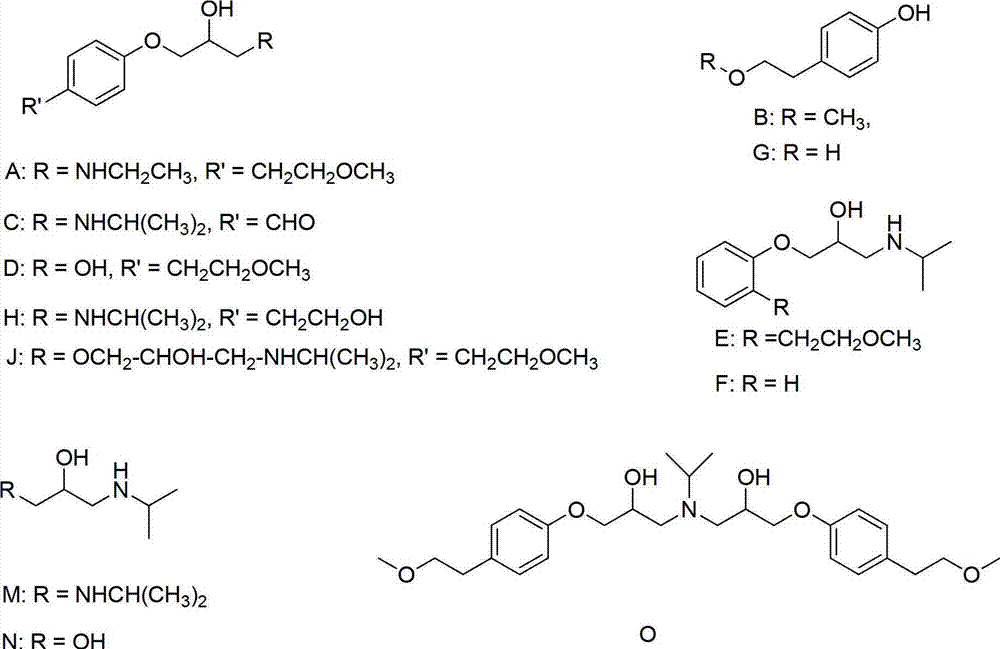
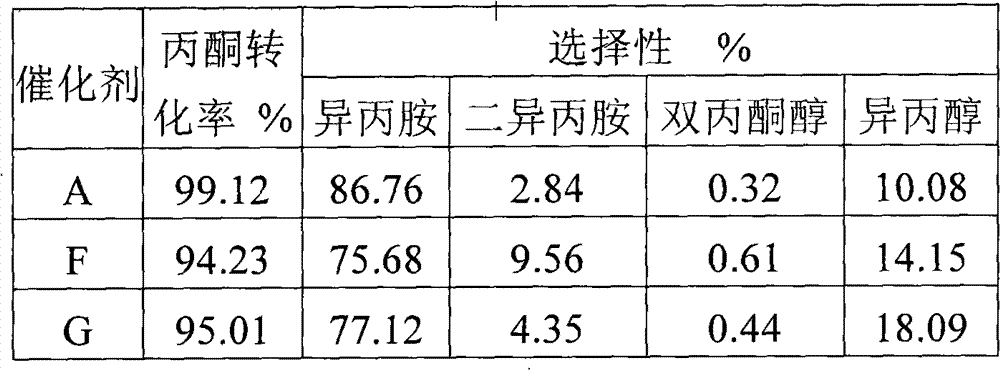
Method for synthesizing isopropamide
ActiveCN101880236AReduce generationSimple production processOrganic compound preparationPreparation by reductive alkylationFatty amineReaction temperature
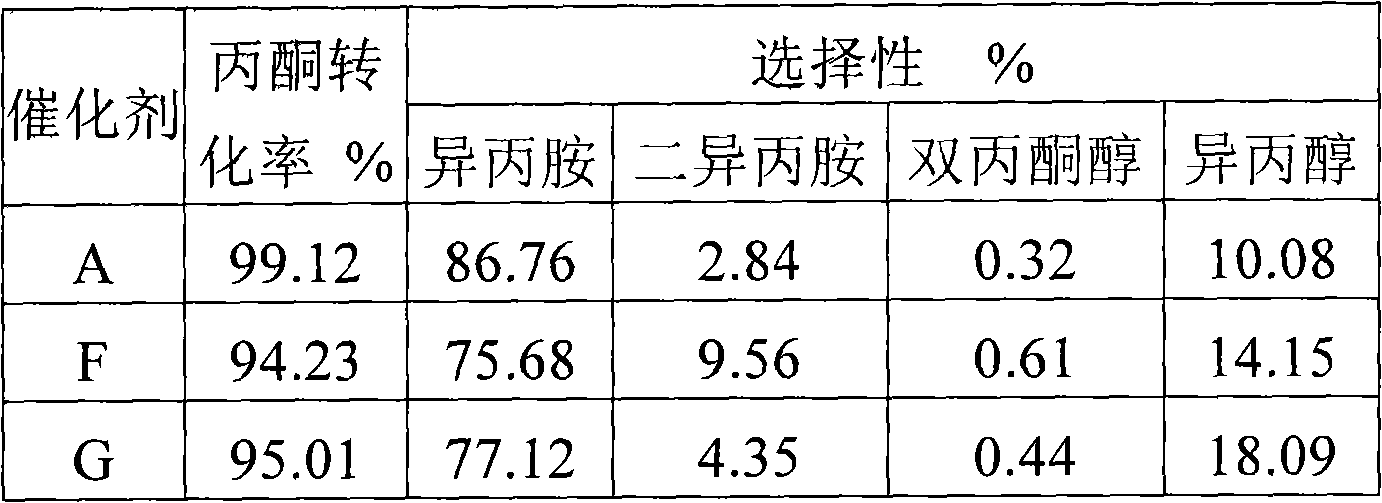
The invention discloses a method for synthesizing isopropamide, belonging to the technical field of synthesizing fatty amine. In order to improve the conversion efficiency and the selectivity of catalysts for synthesizing isopropamide, propanone or isopropanol is used as the raw material, in the presence of hydrogen and a nickel-based catalyst, amination is carried out to synthesize isopropamide under the conditions that the reaction temperature is 110-200 DEG C, the reaction pressure is 0.1-1.0MPa, and the mole ratio of propanone or isopropanol to hydrogen to ammonia is 1:(1-5):(1-5), wherein the catalyst contains a carrier and nickel active components loaded on the carrier, and over 90 percent of the nickel active components are distributed in a region with the depth of 0.7 mm from the surface of the carrier. The method in the invention can ensure that the conversion efficiency of the propanone is improved, has higher propanone conversion efficiency under the condition of same nickel content and better selectivity, reduces the generation amount of diisopropylamine, simplifies the production process flows of the isopropamide, and lowers the production cost of the isopropamide.
Owner:CHINA PETROLEUM & CHEM CORP +1
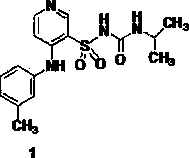
Method for preparing high-purity torasemide and crystal form I thereof
ActiveCN102702089AOvercome the disadvantages of greater toxicityHigh purityOrganic chemistryIsopropamideGranularity
The invention discloses a method for preparing high-purity torasemide and a crystal form I thereof. The method comprises the following steps of: reacting 4-chloro-3-pyridine sulfonamide and m-toluidine serving as raw materials to obtain 3-sulfamoyl-4-(3-methyl phenyl) aminopyridine, refining, and reacting with 1,1'-carbonyldimidazole and isopropamide, directly introducing isopropyl carbamyl, thus obtaining the high-purity torasemide, wherein the purity (high performance liquid chromatography (HPLC)) of the torasemide is more than 99.5 percent. The prepared torasemide has proper granularity, seed crystal is not required for crystal transformation, the torasemide can be directly transformed into the variant crystal form I, and a chemically pure torasemide variant I is obtained. The preparation method is strong in process controllability, easy and convenient to operate and high in reproducibility, and facilitates industrialized production.
Owner:连云港杰瑞药业有限公司
Dicamba aqueous solution
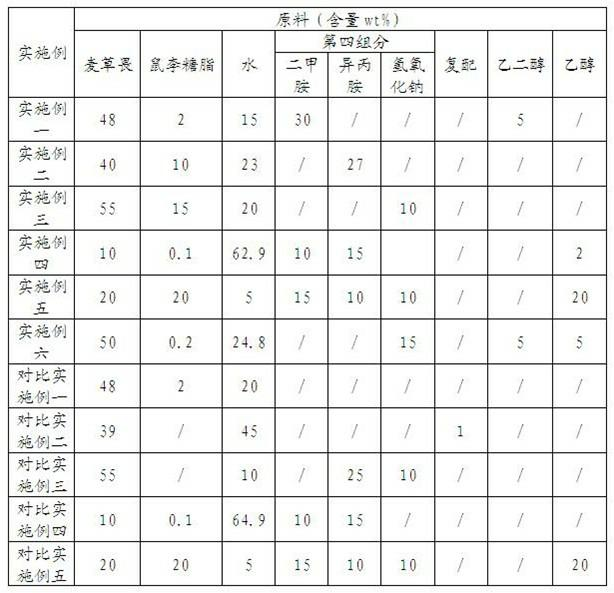
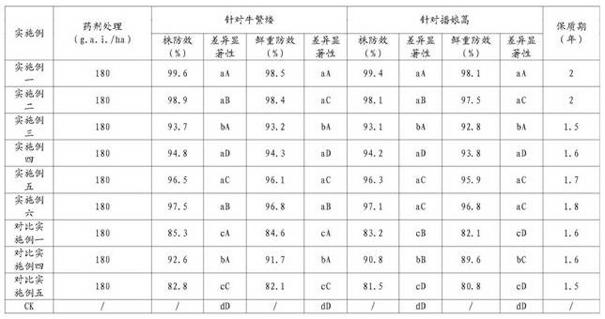
InactiveCN102090394AReduce manufacturing costGood weeding effectBiocideAnimal repellantsIsopropamideSURFACTANT BLEND
The invention relates to a Dicamba herbicide, in particular relating to a Dicamba aqueous solution and a preparation method thereof. The Dicamba aqueous solution comprises the following components in percentage by weight: 10-55% of Dicamba, 0.1-20% of rhamnolipid, 25-35% of a fourth component and the balance of water, wherein the fourth component is one or more of dimethylamine, isopropamide or sodium hydroxide. The preparation method comprises the following steps: after evenly mixing and stirring the Dicamba, the fourth component and water in proportion; then adding the rhamnolipid; and stirring to obtain the Dicamba aqueous solution. A biological surfactant is adopted by the Dicamba aqueous solution, so that the Dicamba aqueous solution is safer and more environmentally-friendly, and the production cost is lowered; after the Dicamba aqueous solution is applied, the weeding effect is ideal, and the Dicamba aqueous solution has the advantages of wide weed killing spectrum, small dosage for each acre and prolonged quality guarantee period.
Owner:ZHEJIANG SHENGHUA BIOK BIOLOGY
Glyphosate isopropamide salt water agent environment-friendly synergist and preparation method thereof
InactiveCN103238625AWith emulsificationDispersive and has regulating functionBiocideAnimal repellantsGlycineBetaine
The invention belongs to a pesticide glyphosate synergist and a preparation method thereof, and particularly relates to a herbicide glyphosate isopropamide salt water agent environment-friendly synergist and a preparation method thereof. The synergist disclosed by the invention comprises the following components in percentage by weight: 20-40% of alkyl glycoside, 40-20% of cationic alkyl glycoside, 12-18% of alkyl glycine betaine, 0.5-1.5% of organic silicon, 2.0-5.0% of ammonium sulfate and the balance of water. The preparation method comprises the steps of uniformly stirring the components in equipment with a stirring function at the temperature of 20-80 DEG C. The glyphosate isopropamide salt water agent environment-friendly synergist is high in quality, efficient and environment-friendly.
Owner:李庆晨
Catalyst for synthesizing isopropamide products, preparation method and application
ActiveCN102172530AHigh catalytic activityGood choicePreparation by reductive alkylationMetal/metal-oxides/metal-hydroxide catalystsMixed oxideIsopropamide
The invention relates to a catalyst for synthesizing isopropamide products by utilizing isopropamide circulation fluid, a preparation method and an application thereof. The catalyst provided by the invention is composed of mixed oxide of Ni, Co, Fe, Cu and Ru and diatomite, and the catalyst has excellent catalytic activity and selectivity and good stability. By adopting the catalyst and process method provided by the invention, the conversion rate of isopropamide circulation fluid is high, the selectivity of mono isopropylamine and diisopropylamine products is high, and the life of the catalyst is long.
Owner:ZHEJIANG XINHUA CHEM
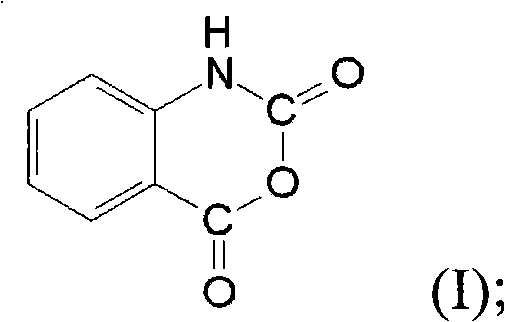
Methods for synthesizing isatoic anhydride and N-isopropyl-2-aminobenzamide
ActiveCN101973956AResponse volume controllableHigh purityOrganic compound preparationCarboxylic acid amides preparationOrganic solvent2-aminobenzamide
The invention provides a method for synthesizing isatoic anhydride, comprising the steps of: dropwise adding a di(trichloromethyl) carbonic ester solution into an o-aminobenzoic acid solution, refluxing for reaction to obtain the isatoic anhydride. The invention further also provides a method for synthesizing N-isopropyl-2-aminobenzamide, comprising the steps of: a) dropwise adding the di(trichloromethyl) carbonic ester solution into the o-aminobenzoic acid solution, and refluxing for reaction to obtain an isatoic anhydride solution; and b) dropwise adding isopropamide into the isatoic anhydride solution, and reacting to obtain the N-isopropyl-2-aminobenzamide. After o-aminobenzoic acid and di(trichloromethyl) carbonic ester react in an organic solvent to generate the isatoic anhydride, the N-isopropyl-2-aminobenzamide can be obtained by directly adding the isopropamide for amidation, without carrying out the steps of extraction, purification, drying and the like on the isatoic anhydride, therefore, operation steps are reduced, production cycle is shortened, and industrialized production is easy to realize.
Owner:HEFEI XINGYU CHEM
Catalyst for preparing isopropamide by aminating acetone as well as preparation method and application thereof
ActiveCN101816938ASimple preparation stepsEasy to operateOrganic compound preparationPreparation by reductive alkylationIsopropamideActive component
The invention discloses a catalyst for preparing isopropamide by aminating acetone as well as a preparation method and application thereof. In order to solve the problems of low conversion rate and selectivity of the traditional isopropamide catalyst, an active component nickel is loaded on an alumina carrier by adopting an in-situ sedimentation method, the content of the nickel is 5-25 by mass percent, and the nickel exists in an amorphous state or a nickel grain is less than 5 nm; and the BET specific surface area of the prepared catalyst is 100-210 m<2> / g. The invention provides the preparation method and the application of the catalyst, has simple preparation method, easy operation and reliable repeatability; and in addition, the catalyst has better acetone conversion rate, selectivity and stability.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing berberine hydrochloride intermittent
InactiveCN107868072AReduce security impactEase of industrial productionOrganic chemistryIsopropamideSynthesis methods
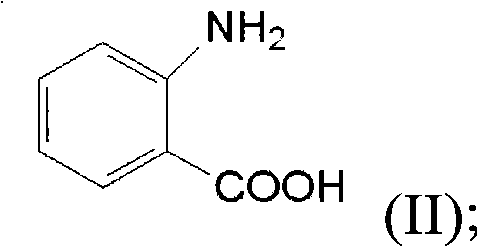
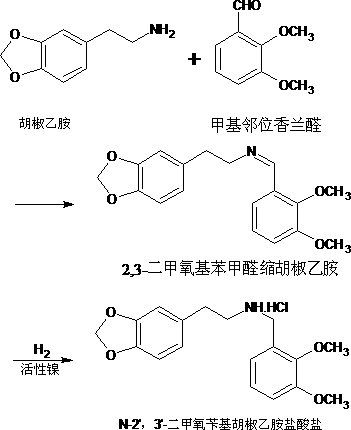
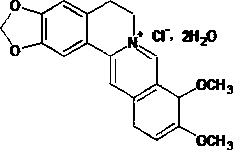
The invention discloses a method for preparing a berberine hydrochloride intermittent. The method comprises the following steps: performing a reduction reaction on an ethanol solution of 2,3-dimethoxybenzaldehyde polycondensed homopiperony lamine (II) and a hydrazine hydrate solution under catalysis of isopropamide, thereby obtaining N-2',3'-dimethoxybenzyl homopiperony lamine (I). Compared witha conventional synthesis method, the method has the advantages of being simple and convenient in synthesis method, high in catalysis activity, easy in product separation and purification, high in product purity, high in yield, small in potential safety hazard, applicable to industrial production, and the like.
Owner:HUAZHONG PHARMA
Method for preparing l-betaxolol hydrochloride
ActiveCN101665441AHigh chemical purityHigh optical purityOrganic compound preparationAmino-hyroxy compound preparationTosylhydrazoneBetaxolol
The invention provides a method for preparing l-betaxolol hydrochloride, which takes p-hydroxy phenylethanol as starting material and obtains the pure l-betaxolol hydrochloride by selectively esterfying alcoholichydroxyl, etherifying phenolichydroxyl, carrying out amination on isopropamide and finally etherifying cyclopropylmethanol to lead HCl to be salified. The invention also provides an important intermediate compound 2-(2S)-3-(4-p-toluene sulfonyloxy ethyl phenoxy)-1,2-propylene oxide which is used for preparing the l-betaxolol hydrochloride, and a preparation method thereof. The invention avoids using bromotoluene cyclopropane which is higher in price, unstable and very irritant; the final products are easy to separate from each other and purify; and the obtained product has higher chemical purity (99.0-100.0%) and optical purity (99.0-100.0%) as well as high yield (total yield is 62%), and is suitable for industrialized production.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
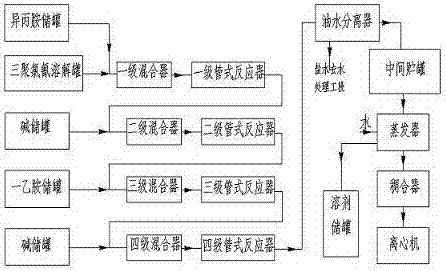
Continuous production process of atrazine
InactiveCN107235924AImprove operational safetyIncrease productivityOrganic chemistryDesolvationEthylamine
The invention relates to the technical field of a continuous production process of atrazine and discloses a continuous production process of atrazine. The continuous production process comprises the following steps: simultaneously pumping a cyanuric chloride solution and isopropamide into a first-stage mixer and a first-stage pipe reactor for reaction; pumping sodium hydroxide into a second-stage mixture, mixing sodium hydroxide with a material liquid to obtain a mixture, and introducing the mixture into a second-stage tubular reactor for reaction; pumping monoethyl amine into a third-stage mixer, mixing monoethyl amine with the material liquid to obtain a mixture, and introducing the mixture into a third-stage pipe reactor for reaction; pumping sodium hydroxide into a four-stage mixer, mixing sodium hydroxide with the material liquid to obtain a mixture, and introducing the mixture into a four-stage pipe reactor for reaction; and after the reaction, separating the material liquid by virtue of an oil-water separator, carrying out a saline dehydration section, carrying out desolvation on an organic phase by virtue of an evaporator, and carrying out centrifugation, and drying, so as to obtain the product. According to the process, the continuous production of atrazine is realized, the production stability, the product quality and the production efficiency are improved, and the yield can reach above 99%; and the process has very high economic and social benefits.
Owner:HEBEI CHENGXIN
Preparation method of related substance E of metoprolol
InactiveCN102964259ALow costHigh yieldOrganic compound preparationEther preparation by ester reactionsPhenethyl alcoholChloroformate
The invention discloses a novel method of a related substance E of metoprolol: 1-isopropamide) propoxyl-3-[2-(2-methoxyl ethyl) phenoxyl-2-propyl alcohol (I). The method comprises the steps of: carrying out a reaction on 2-hydroxyphenylacetic acid (III) and chloro-carbonic ester to generate mixed anhydride and reducing to obtain 2-hydroxyl phenethyl alcohol (IV); carrying out a reaction on a compound (IV) and halogenated benzyl to obtain benzyloxy phenethyl alcohol (V); carrying out a reaction to the compound (IV) and a methylating reagent to form 1-benzyloxy-2-(2-methoxyl ethyl) benzene (VI); hydrocarbonizing the compound (IV) by palladium to obtain 2-(2-methoxyl ethyl); carrying out a reaction on the compound (VII) and epoxy chloropropane in presence of a phase transfer catalyst to obtain 2-((2-(2-methoxyl ethyl) phenoxyl)methyl) ethylene oxide (VIII); and carrying out an isopropamide reaction on the compound (VIII) to obtain the compound (I). The line is low in cost, simple to operate, environment-friendly and highly efficient to obtain products.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Method for synthesizing isopropamide
ActiveCN101880236BReduce generationSimple production processOrganic compound preparationPreparation by reductive alkylationFatty amineReaction temperature
The invention discloses a method for synthesizing isopropamide, belonging to the technical field of synthesizing fatty amine. In order to improve the conversion efficiency and the selectivity of catalysts for synthesizing isopropamide, propanone or isopropanol is used as the raw material, in the presence of hydrogen and a nickel-based catalyst, amination is carried out to synthesize isopropamide under the conditions that the reaction temperature is 110-200 DEG C, the reaction pressure is 0.1-1.0MPa, and the mole ratio of propanone or isopropanol to hydrogen to ammonia is 1:(1-5):(1-5), wherein the catalyst contains a carrier and nickel active components loaded on the carrier, and over 90 percent of the nickel active components are distributed in a region with the depth of 0.7 mm from the surface of the carrier. The method in the invention can ensure that the conversion efficiency of the propanone is improved, has higher propanone conversion efficiency under the condition of same nickel content and better selectivity, reduces the generation amount of diisopropylamine, simplifies the production process flows of the isopropamide, and lowers the production cost of the isopropamide.
Owner:CHINA PETROLEUM & CHEM CORP +1
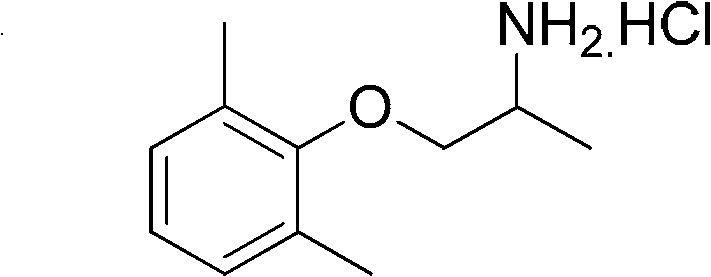
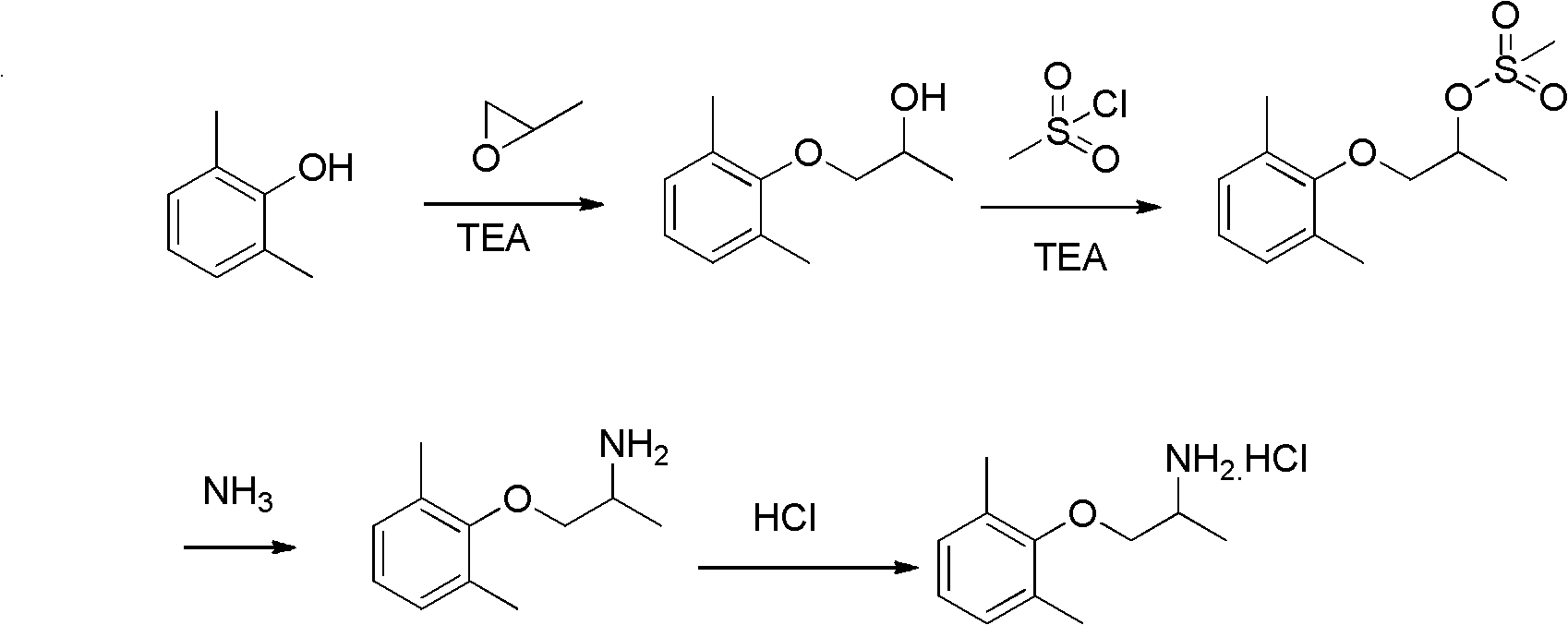
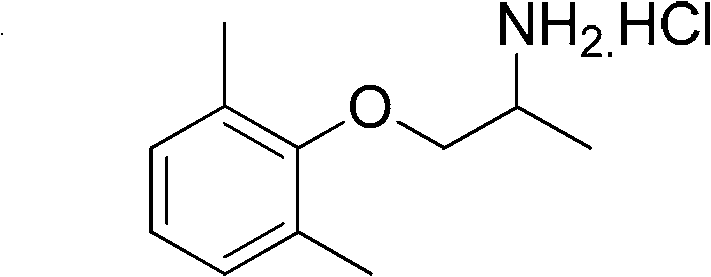
Preparation method of mexiletine hydrochloride
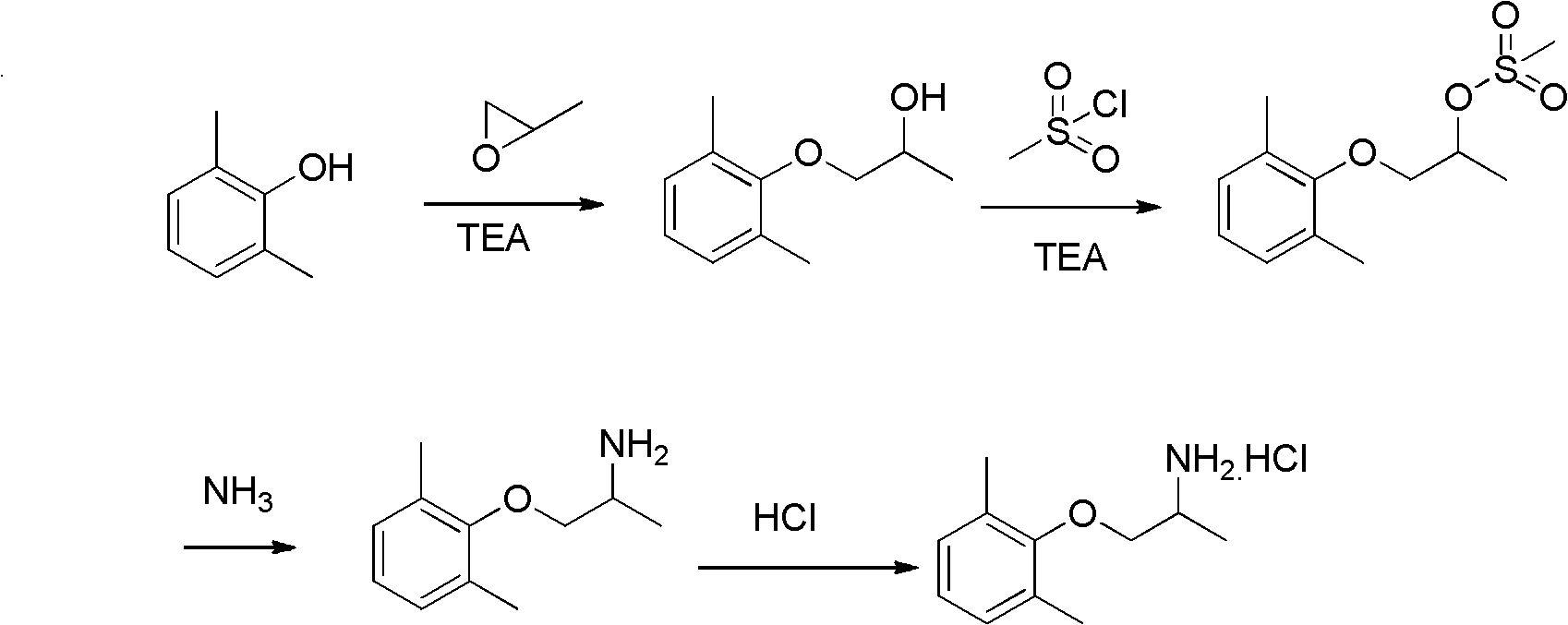
ActiveCN102603543AHigh purityQuality improvementOrganic compound preparationAmino-hyroxy compound preparationIsopropamideMethanesulfonyl chloride
The invention provides a preparation method of mexiletine hydrochloride. The preparation method comprises the following steps of: reacting 2,6-xylenol seriving as a raw material with epoxypropane under the action of a catalysis amount of triethylamine for carrying out hydroxypropylation to obtain 1-(2,6-dimethylphenoxy) isopropanol; carrying out sulfonylation with methanesulfonyl chloride or paratoluensulfonyl chloride based on triethylamine as an acid-binding agent to obtain 1-(2,6-dimethylphenoxy) isopropyl methanesulfonate or 1-(2,6-dimethylphenoxy)isopropyl p-toluenesulfonate; then substituting with ammonia to generate 1-(2,6-dimethylphenoxy) isopropamide; and carrying out salification on 1-(2,6-dimethylphenoxy) isopropamide and hydrochloric acid to obtain mexiletine hydrochloride. The preparation method is simple in reaction operation, available in raw material, easy to post-treat and high in yield and is environment-friendly; intermediate products are unnecessary to purify; and the purity of the obtained product mexiletine hydrochloride can reach more than 99.5%, and the yield is more than 45%.
Owner:FOSHAN PRIZEN MEDICAL TECH
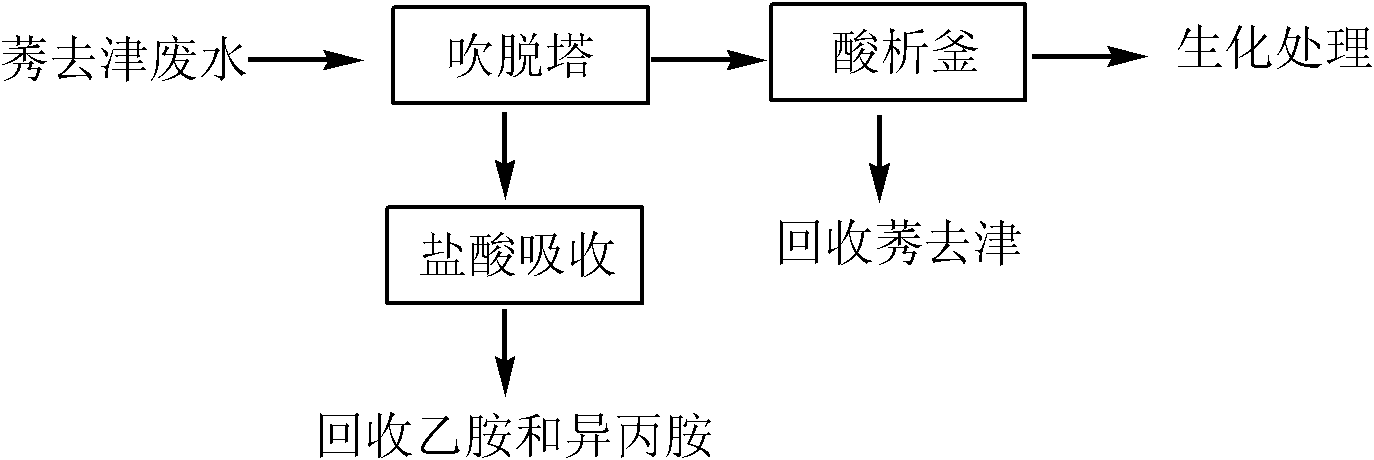
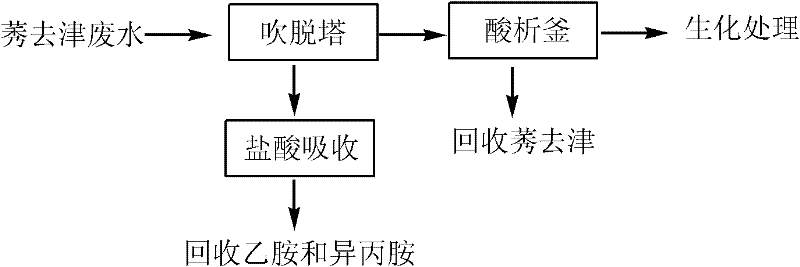
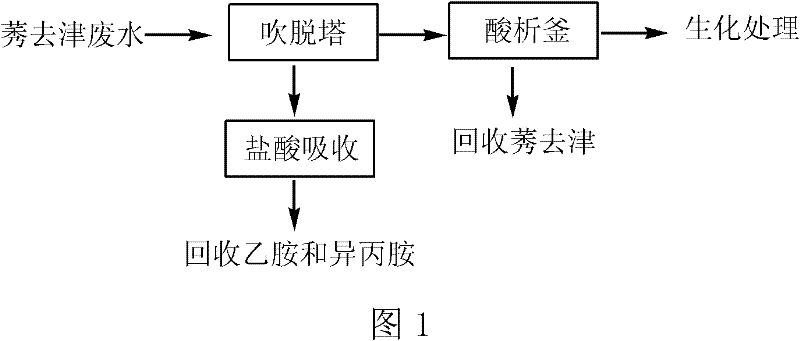
Method for pretreating atrazine production wastewater
ActiveCN102107999ASimple processing methodRealize resource utilizationOrganic chemistryMultistage water/sewage treatmentPretreatment methodChemical oxygen demand
The invention discloses a method for pretreating atrazine production wastewater. The method comprises the following steps of: 1) recovering ethylamine and isopropamide in wastewater through air rectification; and 2) recovering atrazine in the wastewater through acid separation. The pretreatment method is simple and practicable; and by the method, reclamation of the wastewater is realized. Ammonium salt recovered by a blowoff process can be directly applied to production; and the atrazine recovered by an acid separation process can be directly applied to processing atrazine wettable powder. The atrazine content of the wastewater pretreated by the method is less than 3 mg / L and reaches the workshop emission standard of national 'Heterocyclic Pesticide Industrial Water Pollutant Emission Standard'; over 75 percent of chemical oxygen demand (COD) is removed in the process; biochemical practicality is obviously improved; and subsequent biochemical load is reduced.
Owner:中国中化股份有限公司 +2
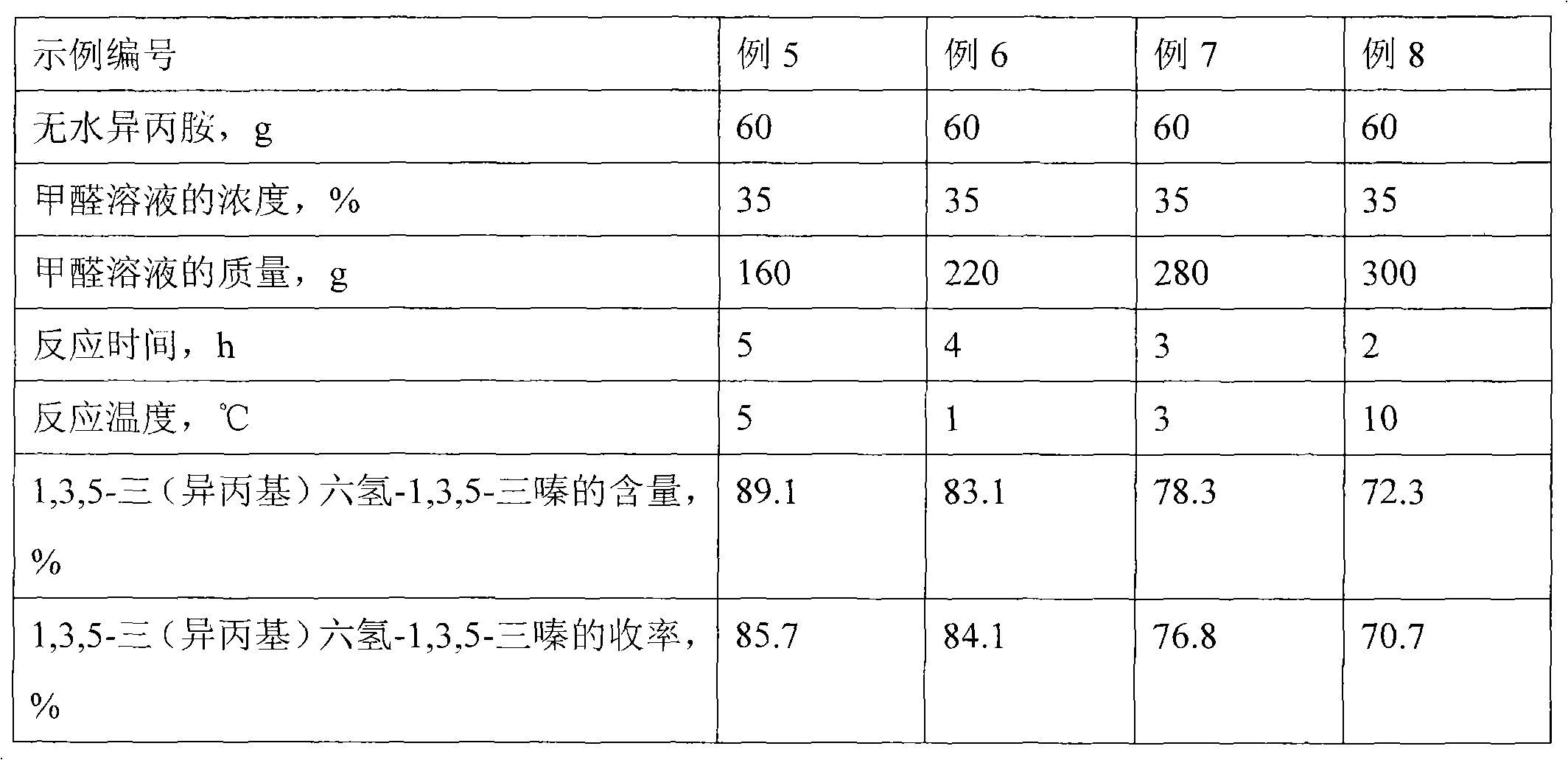
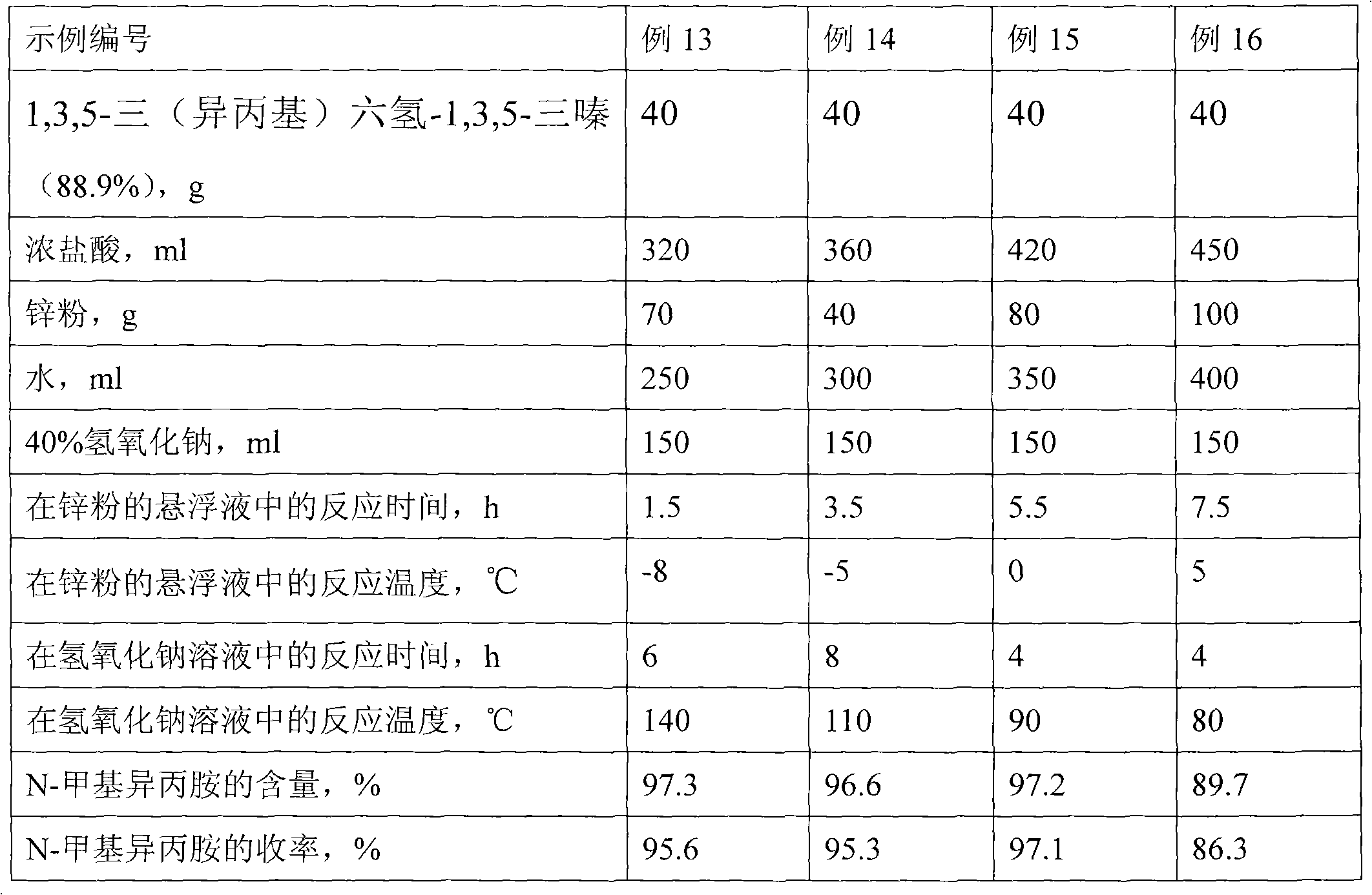
Synthesis method of N-methyl isopropylamine
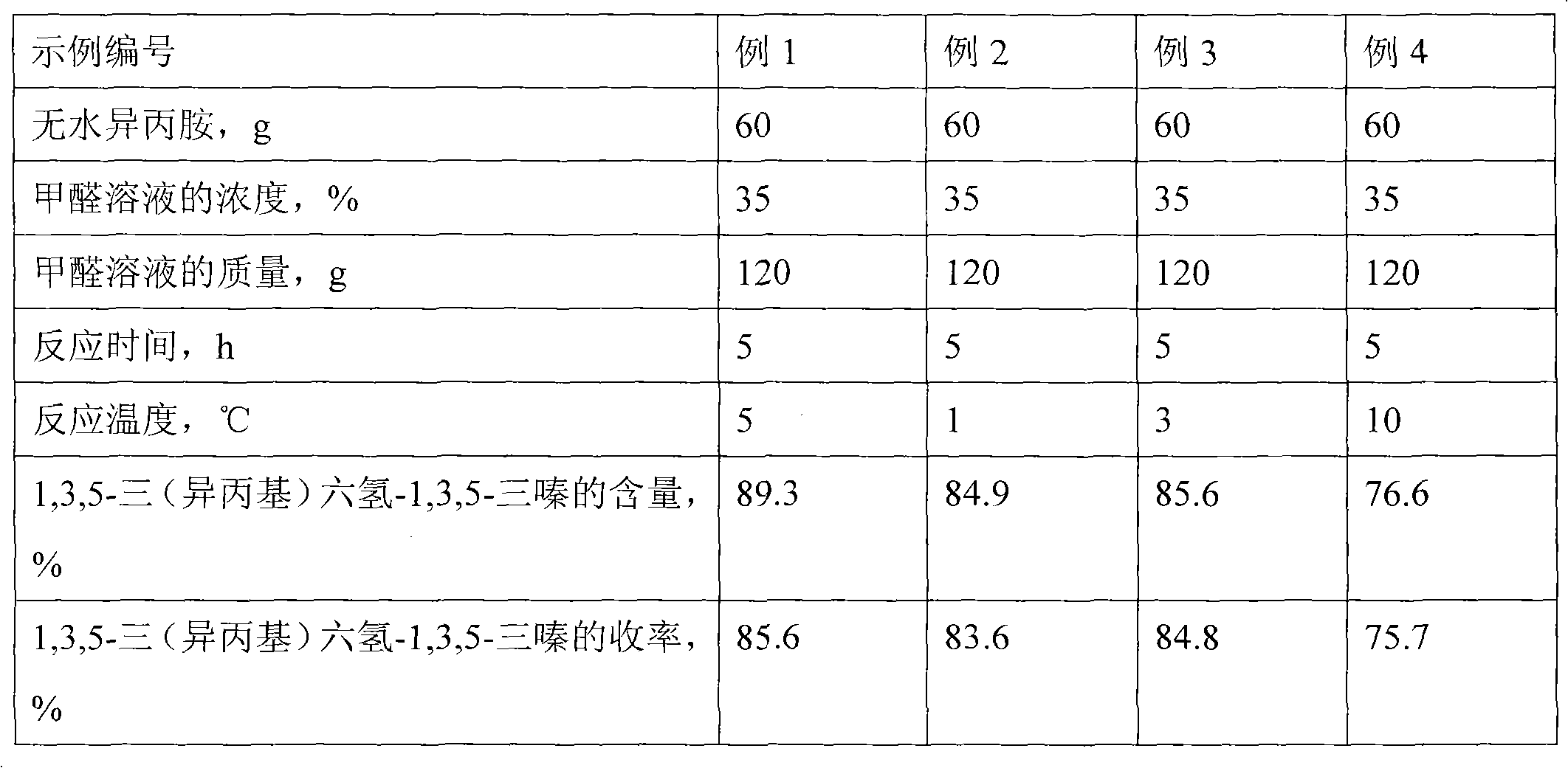
InactiveCN102070461ASimple processImprove conversion rateOrganic compound preparationAmino compound preparationChemical synthesisSynthesis methods
The invention discloses a chemical synthesis method, particularly relates to a preparation method of synthesizing N-methyl isopropylamine by isopropamide. In the synthesis method, the N-methyl isopropylamine is prepared by a Mannich reaction and a decomposition reaction; in the first step, Mannich reaction of anhydrous isopropylamine and formalin solution is carried out; and in the step 2, the decomposition reaction is carried out as follows: respectively adding a reaction product 1,3,5-tri(isopropyl)hexhydro-1,3,5-triazine from the step 1 and concentrated hydrochloric acid in suspending liquid of znic powder and water at the same time, filtering to remove the residual zinc powder after reaction, then dropwise adding filtrate to aqueous sodium hydroxide solution for the decomposition reaction, steaming out the product N-methyl isopropylamine while dripping, and finally, refining, and then obtaining the N-methyl isopropylamine. The synthesis method has the advantages that the process flow is simple, the conversion rate is high, the selectivity is good, the byproducts are less, and the quality of product is stable.
Owner:ZHEJIANG XINHUA CHEM
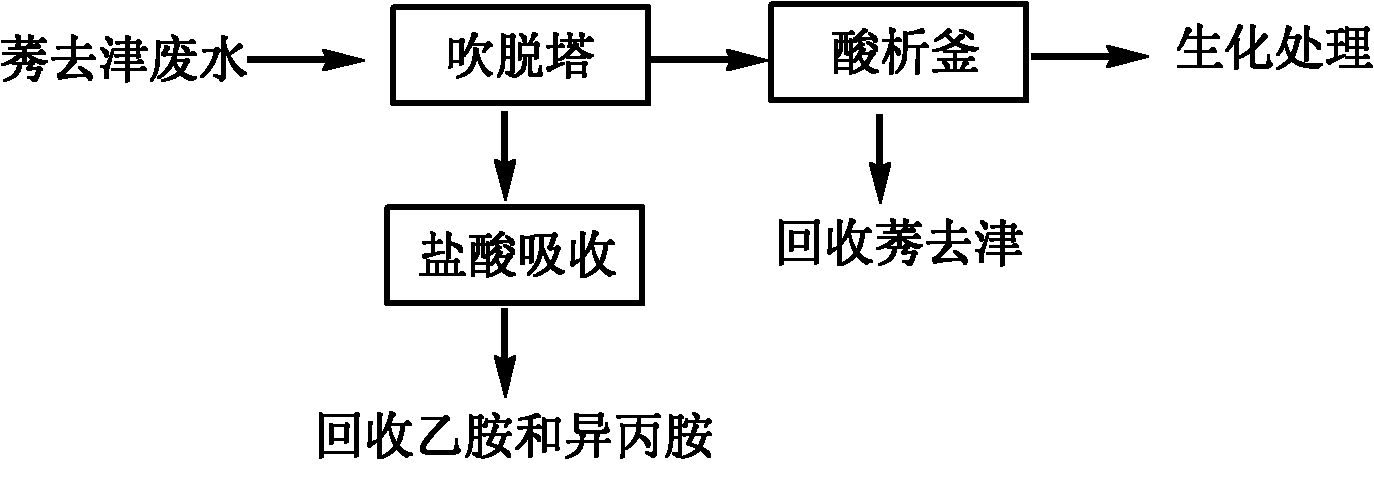
Method for pretreating atrazine production wastewater
ActiveCN102107999BSimple processing methodRealize resource utilizationOrganic chemistryMultistage water/sewage treatmentChemical oxygen demandPretreatment method
The invention discloses a method for pretreating atrazine production wastewater. The method comprises the following steps of: 1) recovering ethylamine and isopropamide in wastewater through air rectification; and 2) recovering atrazine in the wastewater through acid separation. The pretreatment method is simple and practicable; and by the method, reclamation of the wastewater is realized. Ammonium salt recovered by a blowoff process can be directly applied to production; and the atrazine recovered by an acid separation process can be directly applied to processing atrazine wettable powder. The atrazine content of the wastewater pretreated by the method is less than 3 mg / L and reaches the workshop emission standard of national 'Heterocyclic Pesticide Industrial Water Pollutant Emission Standard'; over 75 percent of chemical oxygen demand (COD) is removed in the process; biochemical practicality is obviously improved; and subsequent biochemical load is reduced.
Owner:中国中化股份有限公司 +2
Preparation method of ultrahigh molecular weight polyethylene fine-denier fiber
InactiveCN105369611AGood antibacterialExcellent flame retardantHeat resistant fibresVegetal fibresFiberPolymer science
The invention discloses a preparation method of ultrahigh molecular weight polyethylene fine-denier fiber. The method comprises the step of fiber finishing. According to the step of fiber finishing, 1, 1-2 parts by mass of tetramethyl ammonium bromide, 1.4 parts by mass of silver nitrate, 3.5 parts by mass of N-(phosphono-methyl) glycine isopropamide and 1.7 parts by mass of nanoscale aluminum hydroxide are added into 33 parts by mass of deionized water to be mixed and stirred to be uniform; 2, 3.2 parts by mass of poly alkoxy carboxylic acid salt, 2.8 parts by mass of tea polyphenol, 3.3 parts by mass of rosinyl waterborne polyurethane and 1.2 parts by mass of silver loaded activated carbon are slowly added at the same time for continuous stirring till sufficient and uniform mixing is performed to obtain a finishing solution; 3, hydroxyethyl cellulose fiber is padded in the finishing solution, taken out and dried at constant temperature. The preparation method of the ultrahigh molecular weight polyethylene fine-denier fiber comprises the step of fiber finishing, and the finished hydroxyethyl cellulose fiber has the excellent antibacterial, anti-flaming and anti-static performance.
Owner:CHANGSHU SUPERFIBER
Treating agent for removing sulfur dioxide, sulfur trioxide and hydrogen sulfide from tail gas of oil burning boiler
InactiveCN105148694AStrong complexing abilityFast precipitationDispersed particle separationCyclohexanoneBenzoyl bromide
The invention relates to a treating agent for removing sulfur dioxide, sulfur trioxide and hydrogen sulfide from tail gas of an oil burning boiler. The treating agent is prepared by compounding following components: 2-ethoxy benzaldehyde, 4-methoxy benzyl bromide, cyclohexanone, N,N-diethylethanamine, 3-butene-1-alcohol, 1-tetralone, tricresyl phosphate, N,N-dimethyl trimethylene diamine, 2-methoxy-5-fluorouracil, isopropamide, N-butyloxycarboryl-3-piperidone, ethyl chlorooxoacetate, N,N-dimethyl methylamine, 2-bromine-4'-methylacetophenone, quinoline-2-formaldehyde, absolute ethyl alcohol, and the like. The treating agent is strong in complexing power with target matters, the speed of forming complex and sediment is high, the removal rate can reach 99%, the cost is low, the dosage is less, no scaling is formed and no damage is caused to the environment.
Owner:叶澄
Weed killer
InactiveCN107018987AThe weed control effect is more thanFast weed killingBiocideDead animal preservationCompound aBensulfuron methyl
The invention discloses a weed killer. The weed killer is prepared from 2-amino-3-chlorobenzoate methyl ester, bensulfuron methyl, 2,3-difluoro-5-chloropyridine and 2-chloro-4-diethylin-6-isopropamide-1,3,5-triazine, acetochlor emulsifiable concentrate and atrazine. The weed killer is prepared by compounding a plurality of weed-killing chemical preparations, and has the advantages of stable property, good weed killing effect, small dosage, and no pollution on soil after compounding; the use effect of the weed killer is not influenced by the limits of the types of crops and weeds. The weed killer is suitable for killing weeds.
Owner:王亮
Ternary weeding composition with glyphosate
InactiveCN107347907AReasonable compositionGood weeding effectBiocideAnimal repellantsSuspending AgentsBinary compound
The invention discloses a ternary weeding composition with glyphosate and application of the ternary weeding composition. The ternary weeding composition consists of effective active components, namely a first active component, including one or more of glyphosate, ammonium glyphosate, glyphosate isopropamide, glyphosate sylvite, glyphosate sodium, and glyphosate dimethylamine, a second active component, including one or more of glufosinate-ammonium and glufosinate-ammonium sylvite, and a third active component, namely quizalofop-p-ethyl, wherein the mass ratio of the three active components is (15-45):(1-20):(1-20), preferably is (25-35):(5-15):(1-10), further preferably is 30:10:1, 30:10:3 or 30:10:5. The composition can be prepared into agriculturally allowable suspending agents, dispersible oil suspending agents, wettable powder and water dispersible granules. The composition disclosed by the invention is reasonable in formula and good in non-farmland weeding effect; and the activity and the weeding effects of the active component are not simply overlapped, and compared with a conventional single preparation or a binary compounded preparation, the ternary weeding composition has a remarkable synergic effect in addition to a remarkable weeding effect, is small in dosage, wide in weeding spectrum and lo win prevention and treatment cost.
Owner:SHAANXI SUNGER ROAD BIO SCI
Synthesis technology of bisoprolol
InactiveCN101898972AShorten the timeSimple extraction processOrganic compound preparationChemical recyclingDistillationBisoprolol
The invention relates to a synthesis technology of bisoprolol. The technical scheme of the technology comprises the following steps: (1) using 4-hydroxybenzyl alcohol and isopropoxyethanol to perform the etherification reaction, using strongly acidic styrene type cation exchange resin as catalyst to ensure the alcoholic hydroxyl group etherification reaction to perform under the conditions of room temperature and no solvent; (2) adding sodium hydroxide and epoxychloropropane in the reaction product in the step (1) to ensure phenolic hydroxyl group to perform the etherification reaction; and (3) adding the reaction product in the step (2) in isopropamide, reacting by using boron hydride as catalyst, and preparing bisoprolol. By using the synthesis technology of the invention, the alcoholic hydroxyl group etherification reaction can be performed under the conditions of room temperature and no solvent, the processes of extraction, washing and reduced pressure distillation are simplified; boron hydride is used as catalyst to ensure the amination reaction to perform under a low temperature; the overall yield is increased to above 50%; and the synthesis technology is suitable for industrial production. The solvent used by the technology of the invention can be recycled through distillation; resinic solid acid catalyst can be recycled; and yield and product purity are high.
Owner:张综利
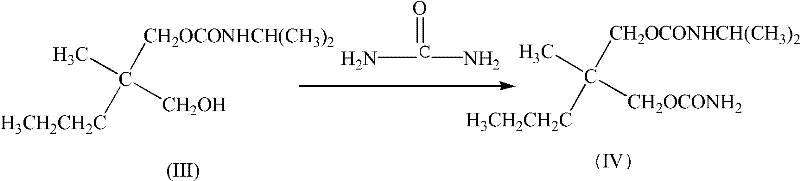
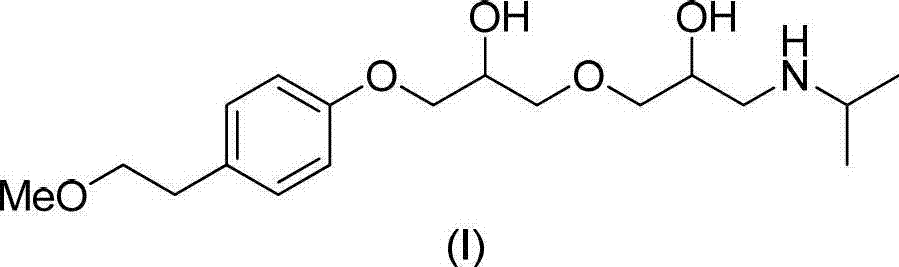
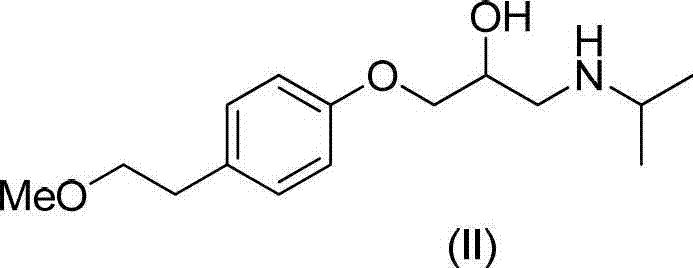
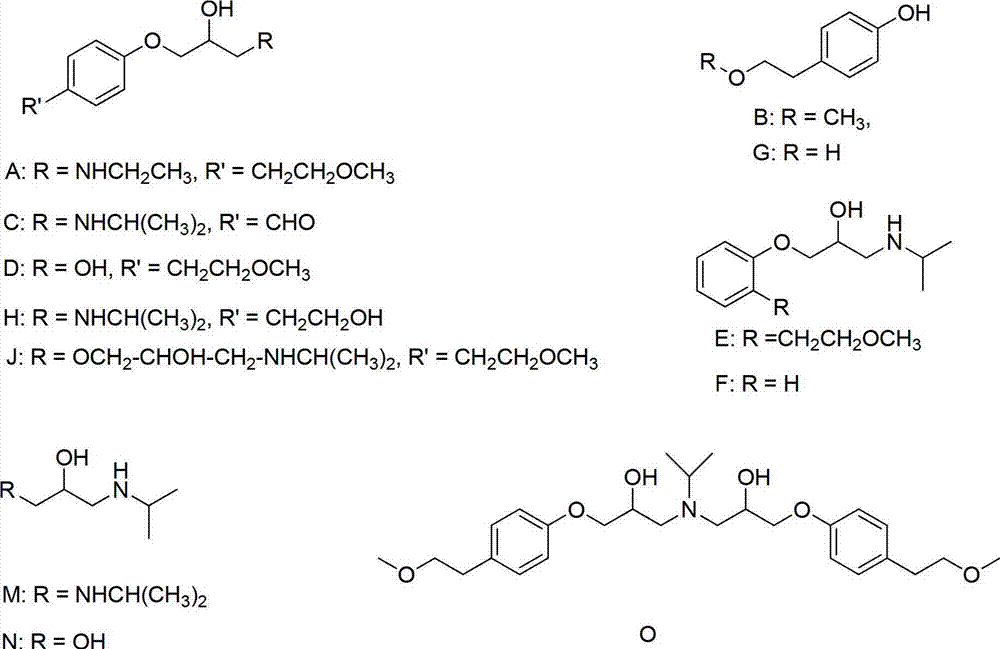
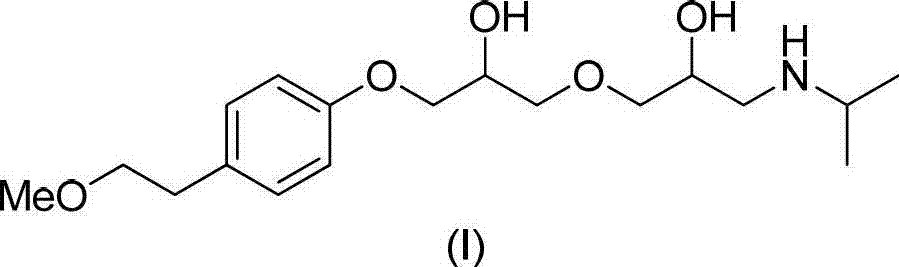
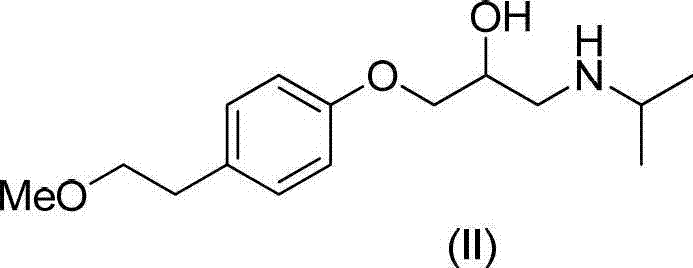
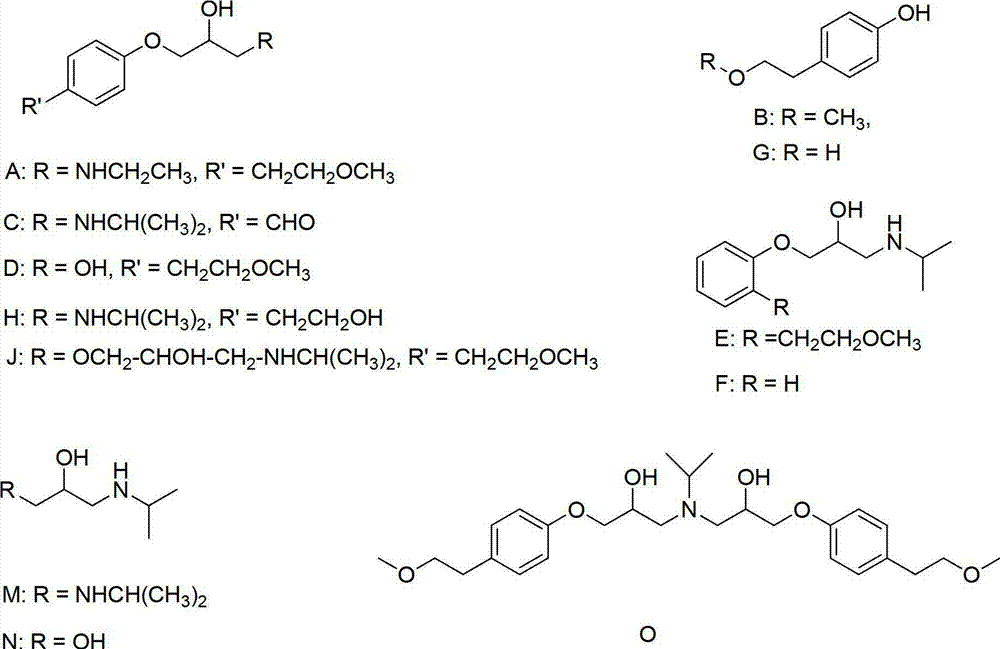
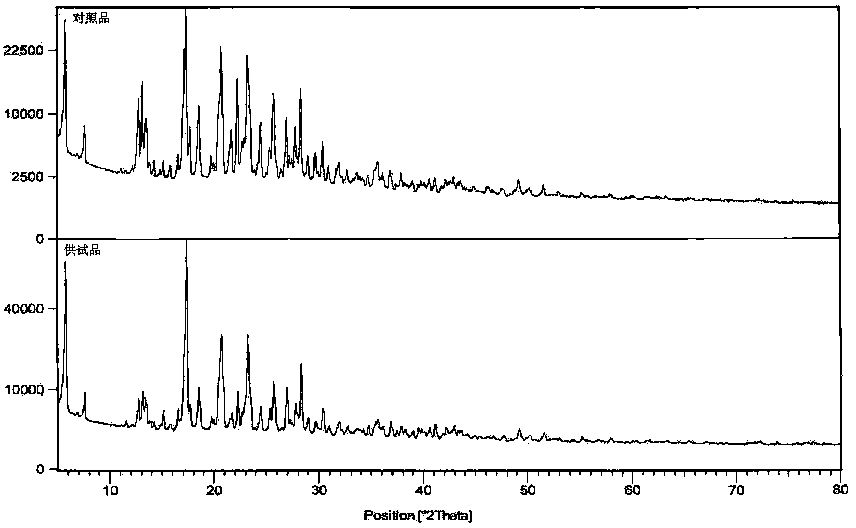
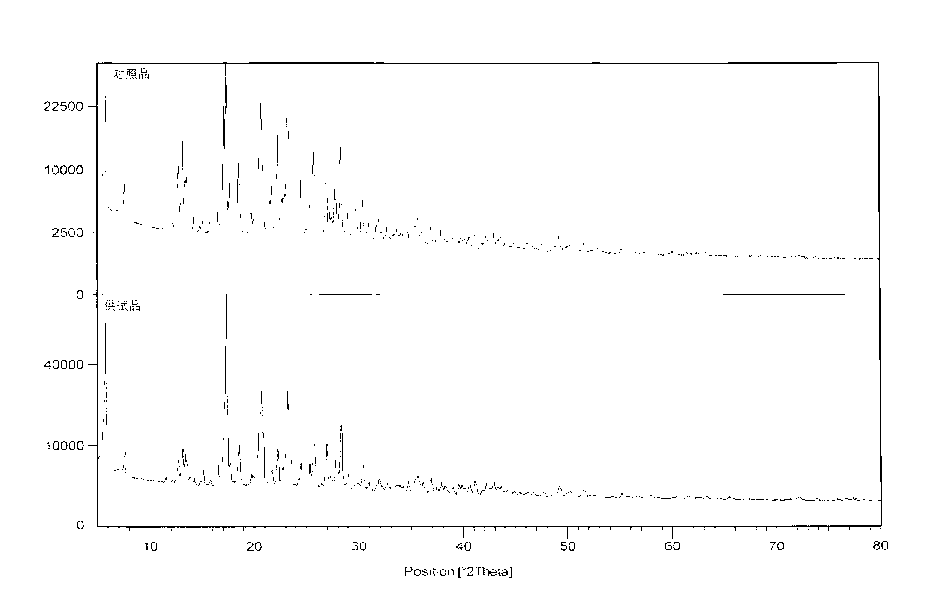
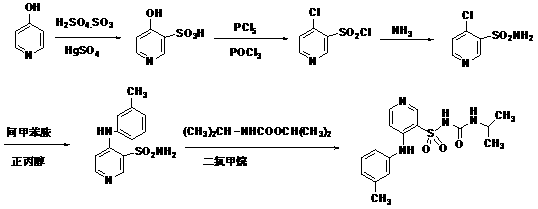
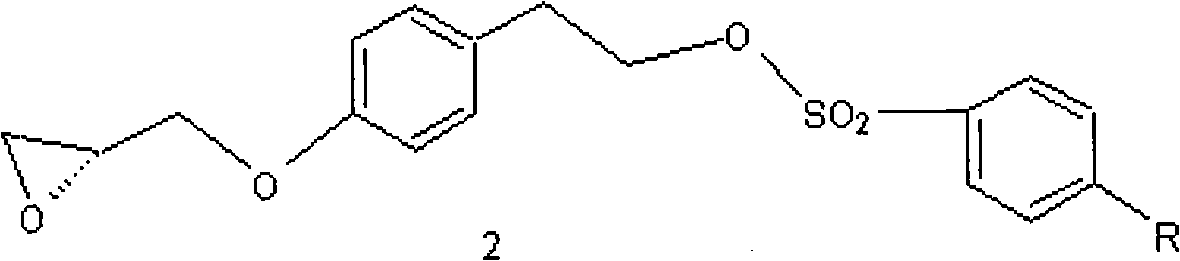
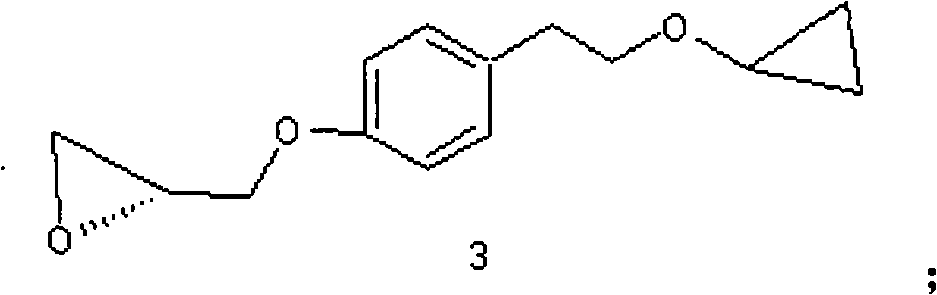
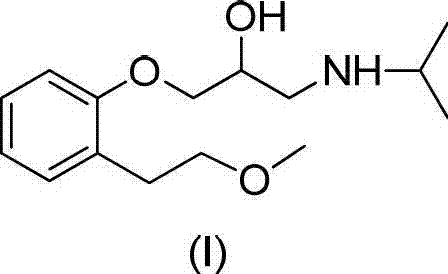
Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride
ActiveCN102329254AEasy to manufactureShort stepsSulfonic acid amide preparationIsopropamideSodium borohydride
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Preparation method of mexiletine hydrochloride
ActiveCN102603543BHigh purityQuality improvementOrganic compound preparationAmino-hyroxy compound preparationMethanesulfonyl chlorideP-toluenesulfonate
The invention provides a preparation method of mexiletine hydrochloride. The preparation method comprises the following steps of: reacting 2,6-xylenol seriving as a raw material with epoxypropane under the action of a catalysis amount of triethylamine for carrying out hydroxypropylation to obtain 1-(2,6-dimethylphenoxy) isopropanol; carrying out sulfonylation with methanesulfonyl chloride or paratoluensulfonyl chloride based on triethylamine as an acid-binding agent to obtain 1-(2,6-dimethylphenoxy) isopropyl methanesulfonate or 1-(2,6-dimethylphenoxy)isopropyl p-toluenesulfonate; then substituting with ammonia to generate 1-(2,6-dimethylphenoxy) isopropamide; and carrying out salification on 1-(2,6-dimethylphenoxy) isopropamide and hydrochloric acid to obtain mexiletine hydrochloride. The preparation method is simple in reaction operation, available in raw material, easy to post-treat and high in yield and is environment-friendly; intermediate products are unnecessary to purify; and the purity of the obtained product mexiletine hydrochloride can reach more than 99.5%, and the yield is more than 45%.
Owner:FOSHAN PRIZEN MEDICAL TECH
Method for separating and purifying diisopropylamine from crude isopropamide
InactiveCN107522622ASimple compositionReduce consumptionMolecular sieve catalystsAmino preparation by hydrogen substitutionIsopropamideMaterial consumption
The invention discloses a method for separating and purifying diisopropylamine from crude isopropamide. The method comprises the following steps: by taking isopropamide as a raw material and taking K / Hbeta zeolite-Al2O3 as a catalyst, compounding diisopropylamine. The conditions for compounding diisopropylamine from isopropamide are as follows: the reaction temperature is 200-300 DEG C, the pressure is from normal pressure to 0.5MPa and the airspeed is 0.2-1.5h. According to the invention, isopropamide is taken as the raw material for continuously compounding diisopropylamine, the original compounding process is simplified, the conversion rate is high, the selectivity is high, the reactant composition is simple, the production time is shortened, the energy is saved, the material consumption is lowered and the production cost is greatly lowered.
Owner:ANHUI HAOYUAN CHEM IND GRP
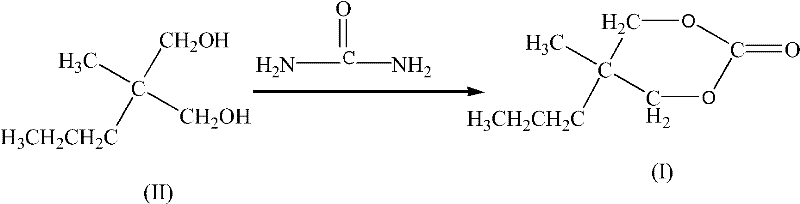
Synthesis method for carisoprodol
InactiveCN102531965ALow costHigh yieldCarbamic acid derivatives preparationOrganic compound preparationCarbamateSynthesis methods
The present invention relates to a preparation method for a compound, and specifically to a synthesis method for carisoprodol. The method is characterized in that the synthesized 5-methyl-5-propyl-1,3-dioxane-2-one is adopted as a raw material; the 5-methyl-5-propyl-1,3-dioxane-2-one and isopropamide are subjected to an aminolysis reaction to generate 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate; the 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate and urea are subjected to a condensation reaction under a catalysis effect of a metal oxide to synthesize the carisoprodol. The method of the present invention has the following advantages that: a new method for synthesis of the carisoprodol from the 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate is provided; the cheap and easily-obtained urea is adopted as the raw material by the method; the yield is stable, and the reaction conditions are mild; the raw material cost is reduced in the synthesis process, and the generated ammonia is easily separated so as to simplify the production process.
Owner:YIYUAN XINQUAN CHEM
Method for preparing metroprolol succinate conforming to European pharmacopoeia and United States pharmacopeia
InactiveCN106866436AReduce Residual IsopropylamineInhibit productionOrganic compound preparationAmino-hyroxy compound preparationIsopropamideDistillation
The invention discloses a method for preparing metroprolol succinate conforming to European pharmacopoeia and United States pharmacopeia. The method comprises the following steps of (1) determining an optimum molar ratio of epoxides and isopropamide through an orthogonal test; (2) carrying out reduced pressure distillation on a reactant so as to remove residual isopropamide as far as possible. According to the method provided by the invention, the residual isopropamide in metroprolol is reduced, a metroprolol succinate solution produced by utilizing the metroprolol synthesized by the method is clear, conforms to standards of European pharmacopoeia / British pharmacopoeia and United States pharmacopeia, and is high in product quality; a postprocessing step is reduced, and the usage of an extracting solvent is avoided, so that not only is the production cost reduced, but also the commercial production is facilitated.
Owner:上海华源医药科技发展有限公司
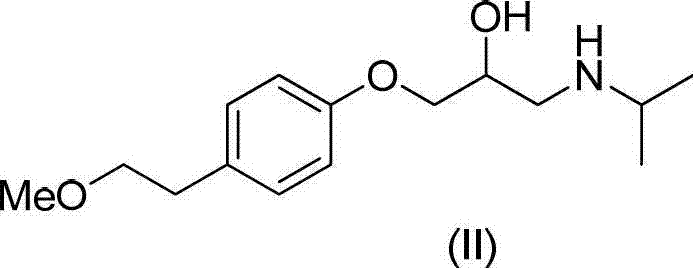
Preparation method of related substance J of metoprolol
ActiveCN102964258AReduce pollutionHigh purityOrganic compound preparationAmino-hyroxy compound preparationEpoxyIsopropylamine
The invention discloses a novel method of a related substance J of metoprolol: 1-(2-hydroxyl-3-isopropamide) propoxyl-3-[4-(2-methoxyl ethyl) phenoxyl-2-propyl alcohol (I). The method comprises the steps of: carrying out a reaction on 4-(2-methly ethyl) phenol (III) and epoxy chloropropane to obtain 1-(2,3-epoxy propoxyl)-4-(2-methoxyl ethyl) benzene (IV); carrying out a reaction on a compound (IV) and allyl alcohol to obtain 1-allyloxy-3-[4-(2-methoxyl ethyl) phenoxyl]-2-propyl alcohol (V); forming 1-[4-(2-methoxyl ethyl) phenoxyl]-3-(2,3-epoxy propoxyl)2-propyl alcohol (VI) under the effect of an oxidizer by the compound (V); and carrying out a reaction on the compound (VI) and isopropylamine to the obtain the compound (I).
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Method for rejecting abnormal plants through resistance identification of insect-resistant and herbicide-resistant transgenic double-gene cottons
InactiveCN102668909AAccurate identificationHigh speedHorticulture methodsPlant genotype modificationHigh resistanceKanamycin
The invention provides a method for rejecting abnormal plants through resistance identification of insect-resistant and herbicide-resistant transgenic double-gene cottons. The method comprises the following steps: spraying a rejecting liquor, which is formed by 41% glyphosate isopropamide of 3.3 ml / L and 500 thousand unit kanamycin of 4000 mg / L, on insect-resistant and herbicide-resistant transgenic double-gene cotton seedlings at the two-leaf and one-bud stage by using a sprayer; and recording spray burn grades of the cotton seedlings one to one when 10 days pass away after spraying the rejecting liquor, wherein a high resistance grade is as follows: the cotton seedlings, 95-100% seed leaves and main leaves of which are not discolored and grow in a normal state, belong to a variety without resistance degradation; a resistance grade is as follows: the cotton seedlings, 90% seed leaves and main leaves of which turn yellow, belong to a variety with degraded resistance; a micro resistance grade is as follows: the cotton seedlings, 80% seed leaves and main leaves of which turn yellow and are locally withered due to water loss, belong to a variety with seriously degraded resistance; and a resistance-free grade is as follows: the cotton seedlings, 70% seed leaves of which are withered and main leaves and interior leaves of which are blackened and rotten, belong to a variety without resistance; and the method for rejecting the abnormal plants through resistance identification of the insect-resistant and herbicide-resistant transgenic double-gene cottons, provided by the invention, has the advantages of accuracy for identification, high speed and simplicity for preparing the rejecting liquor.
Owner:江西省棉花研究所
Preparation method of related substance J of metoprolol
ActiveCN102964258BReduce pollutionHigh purityOrganic compound preparationAmino-hyroxy compound preparationEpoxyIsopropylamine
The invention discloses a novel method of a related substance J of metoprolol: 1-(2-hydroxyl-3-isopropamide) propoxyl-3-[4-(2-methoxyl ethyl) phenoxyl-2-propyl alcohol (I). The method comprises the steps of: carrying out a reaction on 4-(2-methly ethyl) phenol (III) and epoxy chloropropane to obtain 1-(2,3-epoxy propoxyl)-4-(2-methoxyl ethyl) benzene (IV); carrying out a reaction on a compound (IV) and allyl alcohol to obtain 1-allyloxy-3-[4-(2-methoxyl ethyl) phenoxyl]-2-propyl alcohol (V); forming 1-[4-(2-methoxyl ethyl) phenoxyl]-3-(2,3-epoxy propoxyl)2-propyl alcohol (VI) under the effect of an oxidizer by the compound (V); and carrying out a reaction on the compound (VI) and isopropylamine to the obtain the compound (I).
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Method for preparing high-purity torasemide and crystal form I thereof
The invention discloses a method for preparing high-purity torasemide and a crystal form I thereof. The method comprises the following steps of: reacting 4-chloro-3-pyridine sulfonamide and m-toluidine serving as raw materials to obtain 3-sulfamoyl-4-(3-methyl phenyl) aminopyridine, refining, and reacting with 1,1'-carbonyldimidazole and isopropamide, directly introducing isopropyl carbamyl, thus obtaining the high-purity torasemide, wherein the purity (high performance liquid chromatography (HPLC)) of the torasemide is more than 99.5 percent. The prepared torasemide has proper granularity, seed crystal is not required for crystal transformation, the torasemide can be directly transformed into the variant crystal form I, and a chemically pure torasemide variant I is obtained. The preparation method is strong in process controllability, easy and convenient to operate and high in reproducibility, and facilitates industrialized production.
Owner:连云港杰瑞药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/068abe0a-bf82-4c82-8f81-2f82b6e85878/BSA00000184793200011.PNG)
![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/068abe0a-bf82-4c82-8f81-2f82b6e85878/BSA00000184793200021.PNG)
![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/068abe0a-bf82-4c82-8f81-2f82b6e85878/BSA00000184793200022.PNG)