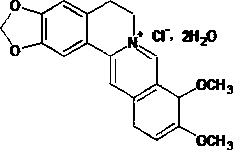
Method for preparing berberine hydrochloride intermittent
A technology for berberine hydrochloride and intermediates, which is applied in the field of preparation of berberine hydrochloride intermediates, can solve the problems of unsuitability for industrialized production, high cost of sodium borohydride, and complicated post-processing processes, and achieves convenient industrialized production and catalytic performance. The effect of high activity and selectivity and low catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
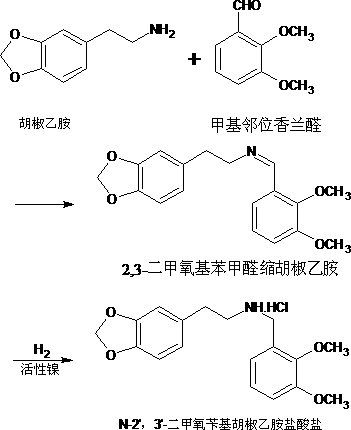
[0025] Add the ethanol solution of 2,3-dimethoxybenzaldehyde piperonyl ethylamine (which contains 200g of 2,3-dimethoxybenzaldehyde piperonyl ethylamine), 1.6g of isopropylamine and 100g of hydrazine hydrate aqueous solution into the reaction flask . React at 50-65°C for 4h. Cool down, concentrate to dryness under reduced pressure, add hydrogen chloride / ethanol solution dropwise to pH 4~5, cool down to below 0°C, crystallize, filter, and dry to obtain N-2',3'-dimethoxybenzylpiperylethylamine Hydrochloride 204g, yield 96.4%, melting point 144.3-145.2°C, HPLC purity 99.1%.
Embodiment 2
[0027] Add 2,3-dimethoxybenzaldehyde piperonyl ethylamine ethanol solution (which contains 2,3-dimethoxybenzaldehyde piperonyl ethylamine 200g), 1g isopropylamine and 120g hydrazine hydrate aqueous solution into the reaction flask. React at 65-75°C for 5h. Cool down, concentrate to dryness under reduced pressure, add hydrogen chloride / ethanol solution dropwise to pH 4~5, cool down to below 0°C, crystallize, filter, and dry to obtain N-2',3'-dimethoxybenzylpiperylethylamine Hydrochloride 203g, yield 95.9%, melting point 144.5-145.6°C, HPLC purity 99.0%.
Embodiment 3
[0029] Add 2,3-dimethoxybenzaldehyde piperonyl ethylamine ethanol solution (which contains 2,3-dimethoxybenzaldehyde piperonyl ethylamine 200g), 2.4g isopropylamine and 60g hydrazine hydrate aqueous solution into the reaction flask . React at 30-40°C for 5h. Cool down, concentrate to dryness under reduced pressure, add hydrogen chloride / ethanol solution dropwise to pH 4~5, cool down to below 0°C, crystallize, filter, and dry to obtain N-2',3'-dimethoxybenzylpiperylethylamine Hydrochloride 206g, yield 97.3%, melting point 144.3-145.4°C, HPLC purity 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com