Synthesis method of N-methyl isopropylamine
A technology of methyl isopropylamine and a synthesis method, which is applied in the field of preparation of N-methyl isopropylamine, can solve the problems of serious equipment corrosion, poor product selectivity, large one-time investment, etc., and achieves low production cost, low pollution and side effects. The effect of less product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of 1,3,5-tri(isopropyl)hexahydro-1,3,5-triazine
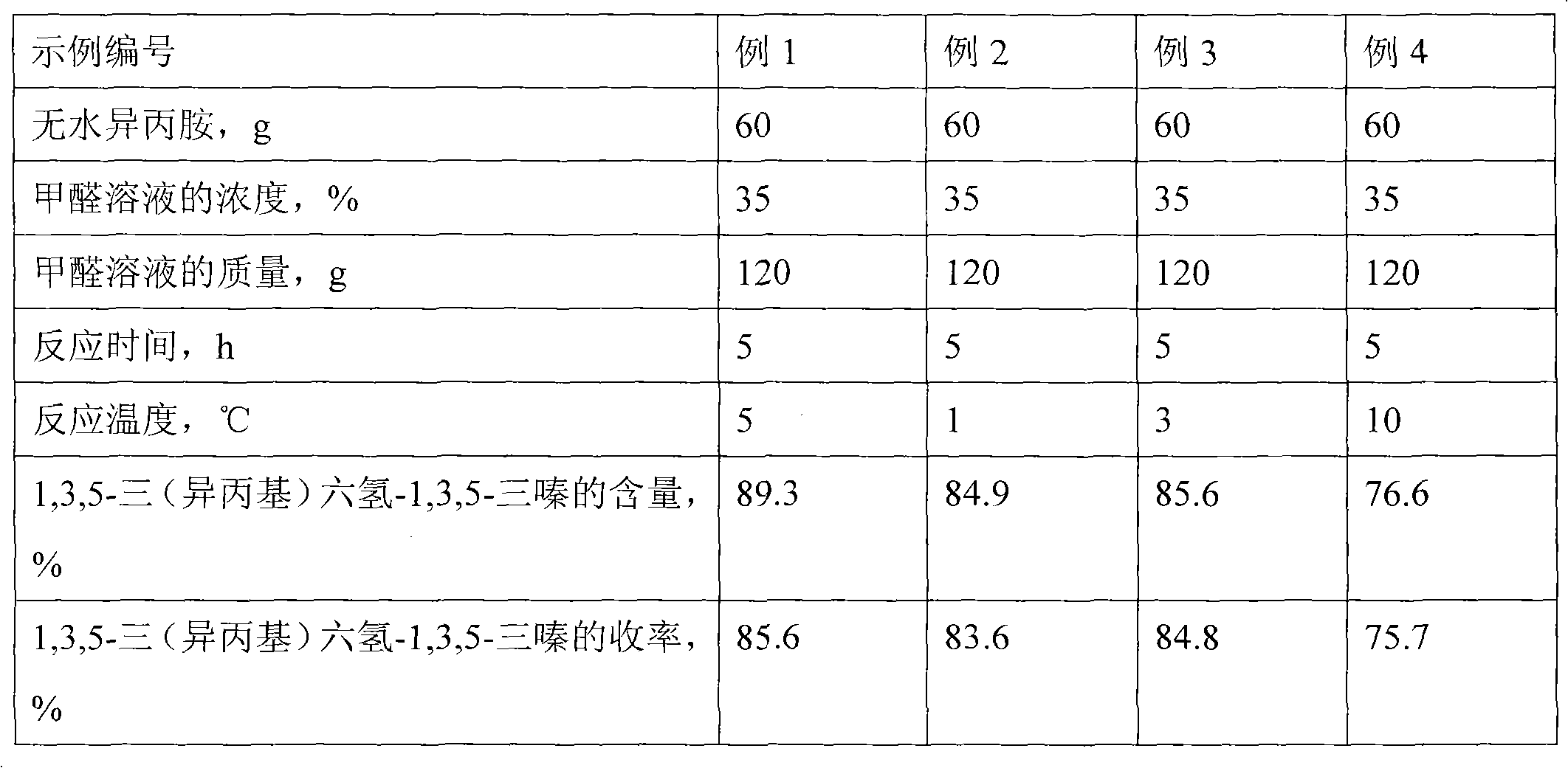
[0015] In a 250ml four-necked flask equipped with a stirrer, a reflux condenser, a thermometer and a dropping funnel, add 60 g of anhydrous isopropylamine, use an ice-water bath to control the temperature at 5°C, stir rapidly, and add dropwise from the dropping funnel 35% formaldehyde solution 120g, in the case of rapid stirring, after 1h dripping, continue stirring in ice water bath for 4h, after the reaction is completed, the oil layer is separated, and dried with anhydrous magnesium sulfate for 12h to obtain 1,3,5-tris (Isopropyl) hexahydro-1,3,5-triazine is genuine product, with a content of 89.3% and a yield of 85.6%.
Embodiment 2-8
[0017] Preparation of 1,3,5-tri(isopropyl)hexahydro-1,3,5-triazine
[0018] The effect of reaction temperature on the reaction
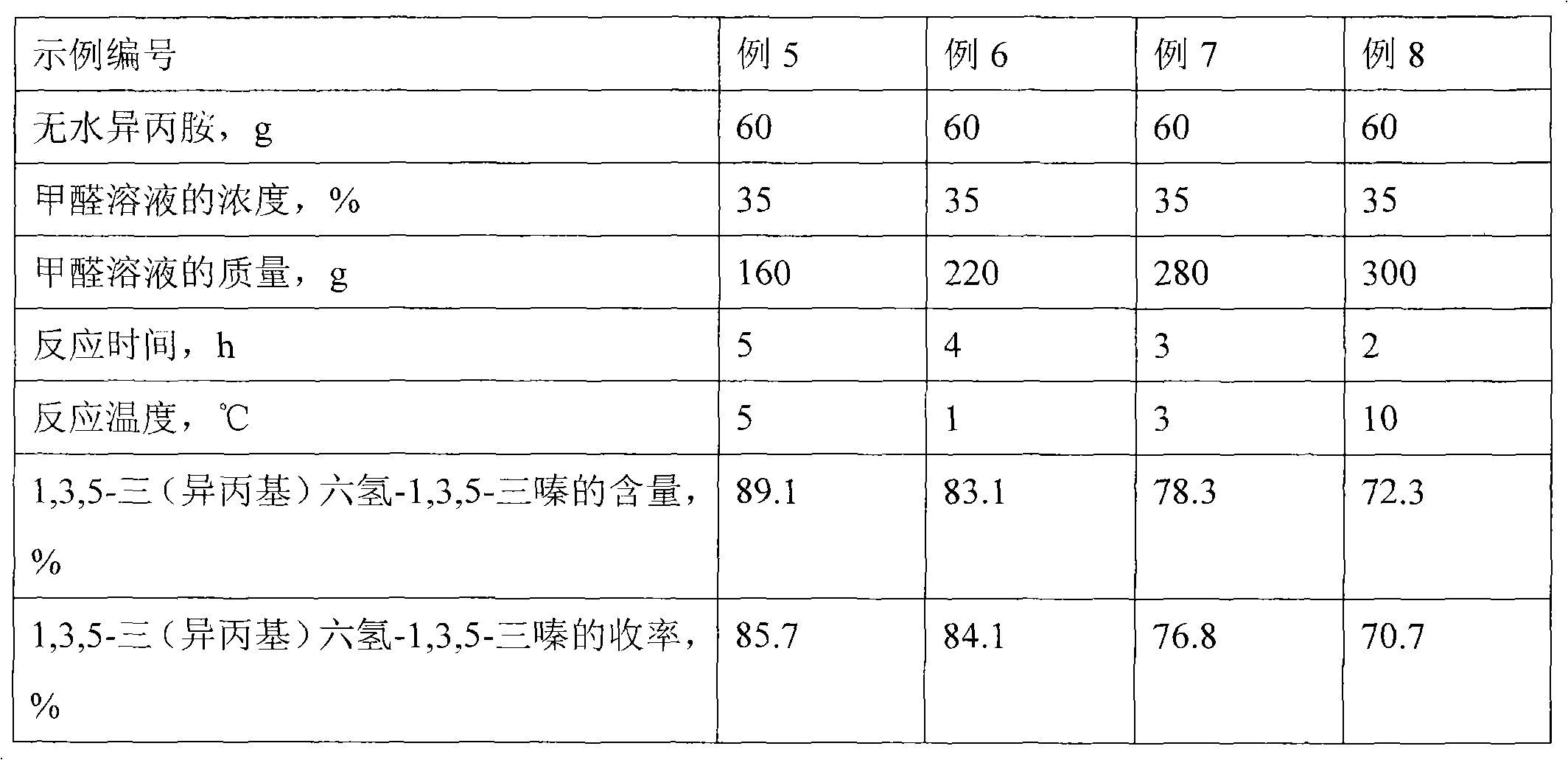
[0019] The difference from Example 1 is that the reaction temperatures in Examples 2-4 are 1°C, 3°C, and 10°C, respectively. Other reaction conditions are the same as those in Example 1. The results are listed in Table 1 after chromatographic analysis.
[0020] Table 1 Influence of reaction temperature on reaction
[0021]
[0022] Table 2 Effects of different reaction conditions on the reaction
[0023]
Embodiment 9
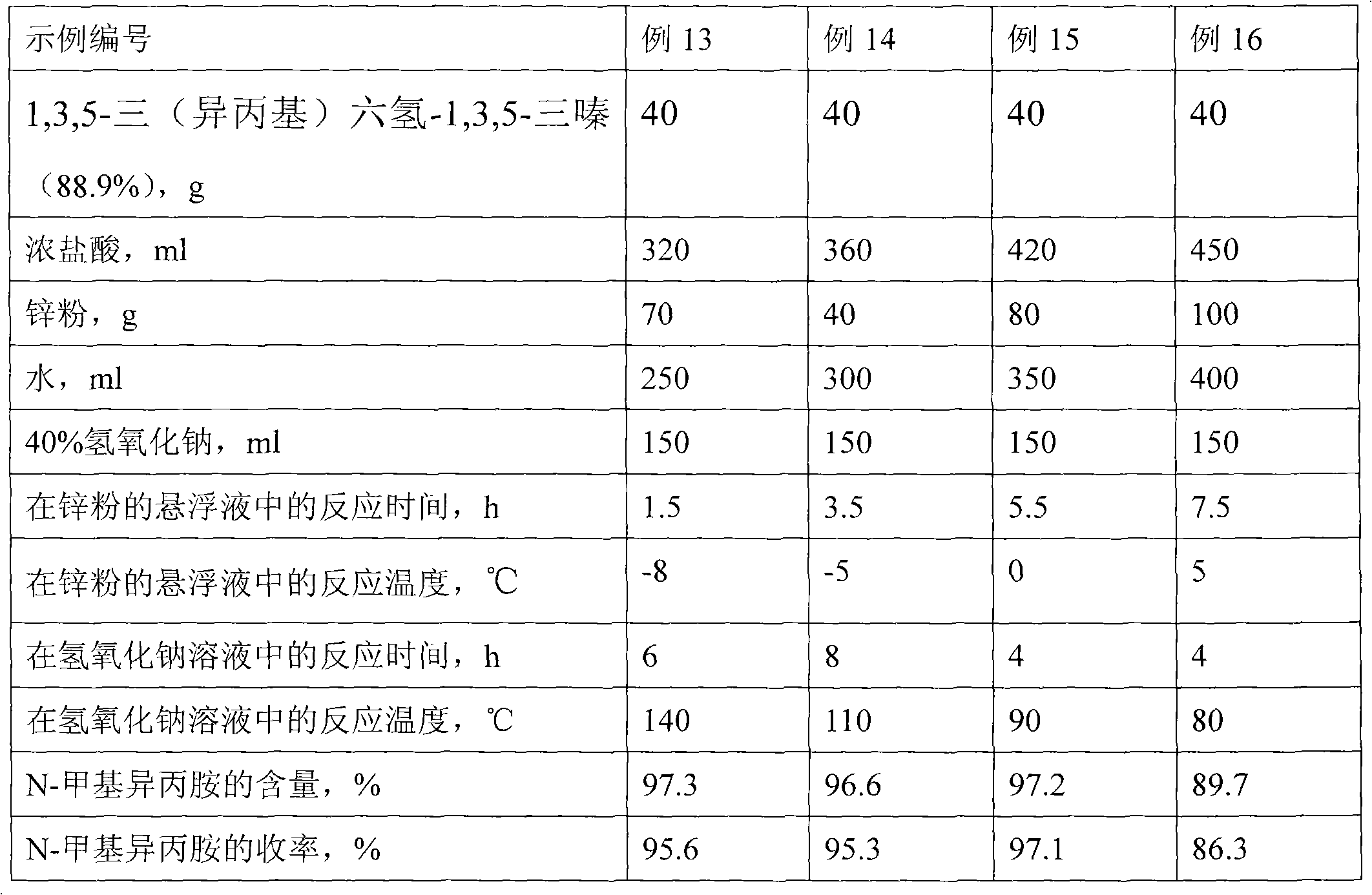
[0025] Preparation of N-methylisopropylamine
[0026] 70g of zinc powder and 200ml of water were put into the three-necked flask, the temperature was controlled at -5°C, and 40g of 1,3,5-tris(isopropyl)hexahydro-1 was dropped simultaneously with a dropping funnel under strong stirring. 3,5-triazine and 320ml of concentrated hydrochloric acid are controlled to be dripped within 0.5h. After the dripping is completed, stirring is continued for 1h, and the remaining zinc powder is removed by filtration. Then 150ml of 40% sodium hydroxide solution was put into the three-necked flask of the reactive distillation unit, the temperature was controlled at 90°C, and the filtrate was slowly added dropwise with a dropping funnel under strong stirring, and the dropping was completed within 2 hours. Then continue to stir the reaction for 2h, and steam out the product N-methylisopropylamine while adding dropwise. Finally, after re-rectifying the N-methylisopropylamine obtained by reactive di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com