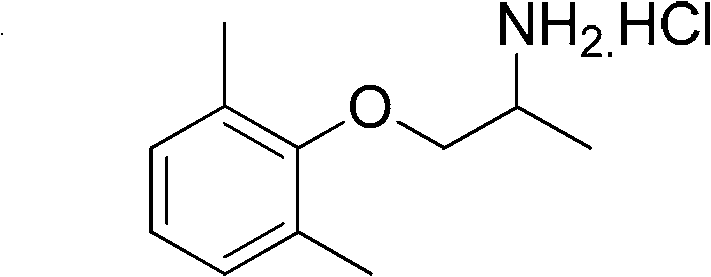
Preparation method of mexiletine hydrochloride
A technology of mexiletine hydrochloride and its production method, which is applied in the production field of mexiletine hydrochloride, can solve problems such as the existence of production technology, hidden dangers of industrial production safety, and hidden dangers of safety, and achieve low production costs, significant economic value, and improved quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
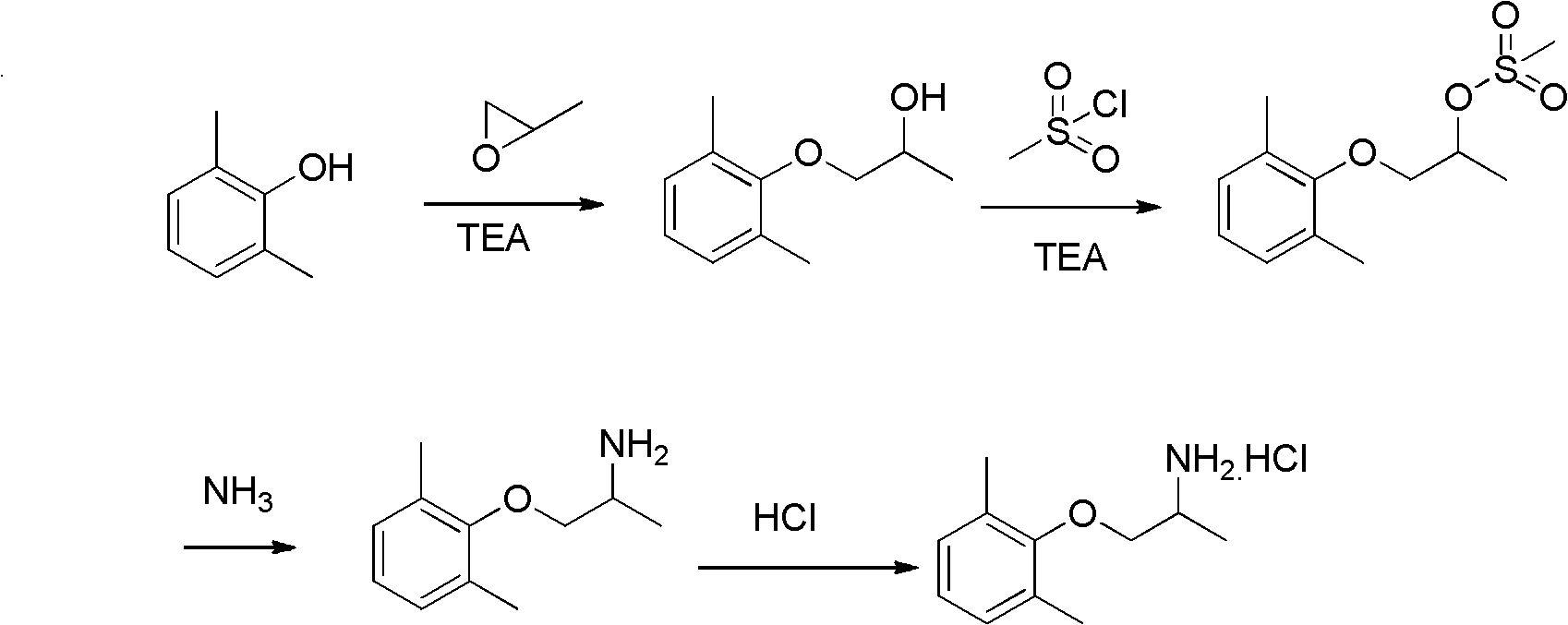
[0029] Step 1: Weigh 1.22Kg of 2,6-dimethylphenol, 580g of propylene oxide and 70g of triethylamine, dissolve in 2.5L of methanol, heat to 60℃ in a 5L reactor, and stir for 6 hours to react End. The solvent methanol and triethylamine were distilled off under reduced pressure at 40°C, and the temperature was increased to collect the fraction with a pressure of 2mmHg and 110~112°C to obtain a colorless and transparent liquid, namely 1-(2,6-dimethylphenoxy) Isopropanol was 1.66 Kg, and the yield was 92.2%.
[0030] NMR data: 1 H-NMR (CDCl3, 400MHz): δ (ppm) = 7.02 (d, 2H, J = 7.2 Hz), 6.92-6.95 (m, 1H), 4.18-4.27 (m, 1H), 3.72-3.75 (m, 1H), 3.63-3.67 (m, 1H), 2.63 (d, 1H, J=3.2 Hz), 2.29 (s, 6H), 1.27 (d, 3H, J=6.4 Hz).
[0031] Step 2: Dissolve 1.66Kg of 1-(2,6-dimethylphenoxy)isopropanol and 1.06Kg of methanesulfonyl chloride in 5L of dry dichloromethane, cool to 0℃ in an ice water bath, and at 0℃, Add 1.02Kg of triethylamine to the system dropwise. After the addition is complete...
Embodiment 2
[0045] Step 1: Weigh 1.22Kg of 2,6-dimethylphenol, 726g of propylene oxide and 100g of triethylamine, dissolve it in 2.5L of methanol, heat it to 80°C in a 5L reactor, and stir for 3 hours to react. End. The solvent methanol and triethylamine were distilled off under reduced pressure at 40°C, and the temperature was increased to collect the fraction with a pressure of 2mmHg and 110~112°C to obtain a colorless and transparent liquid, namely 1-(2,6-dimethylphenoxy) The isopropanol was 1.72 Kg, and the yield was 95.6%.
[0046] Step 2: Dissolve 1.72Kg of 1-(2,6-dimethylphenoxy)isopropanol and 1.26Kg of methanesulfonyl chloride in 6L of dry toluene, cool to 0℃ in an ice water bath, and add to the system at 35℃ 1.11Kg of triethylamine was added dropwise to the medium. After the addition, continue to stir for 45 minutes. A large amount of white solid precipitates out. Filter, first wash the filter cake with toluene, combine the filtrate and washing liquid to obtain the organic phase, ...
Embodiment 3
[0059] Step 1: Weigh 1.22Kg of 2,6-dimethylphenol, 878g of propylene oxide and 73.2g of triethylamine, dissolve them in 2.5L of ethanol, heat to 100°C in a 5L reactor, and stir for 2 hours. The reaction is over. The solvent ethanol and triethylamine were distilled off under reduced pressure at 40°C, and the temperature was raised to collect the fraction with a pressure of 2mmHg and 110~112°C to obtain a colorless and transparent liquid, namely 1-(2,6-dimethylphenoxy) Isopropanol was 1.58Kg, and the yield was 87.8%.
[0060] Step 2: 1-(2,6-Dimethylphenoxy)isopropanol 1.58Kg and methanesulfonyl chloride 1.50Kg are dissolved in 6L of dry dichloromethane, cooled to 0℃ in an ice water bath, at 5℃, 1.33Kg of triethylamine was added dropwise to the system. After the addition, the stirring was continued for 60 minutes. A large amount of white solid precipitated out. Filter. First wash the filter cake with dichloromethane. Combine the filtrate and the washing solution to obtain an organi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com