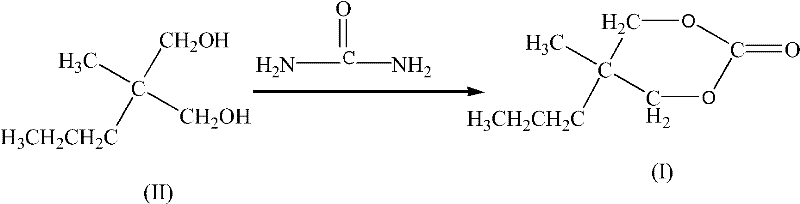
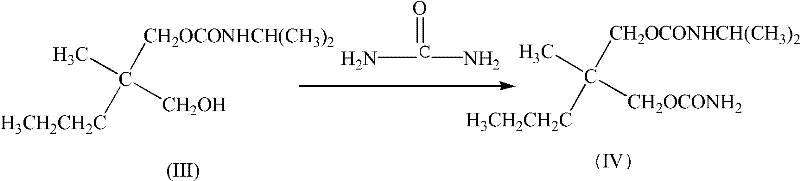
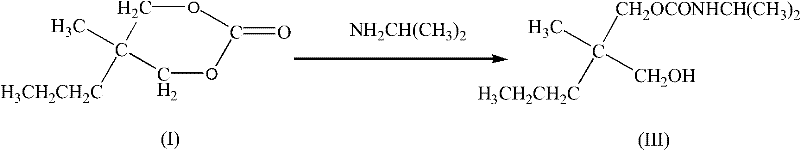Synthesis method for carisoprodol
A synthetic method and the technology of carisoprodol, applied in the field of compound preparation, can solve problems such as difficult control, harsh reaction conditions, and expensive raw material cyanate, and achieve easy separation, stable route yield, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The ratio of 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate: urea: activated ZnO is 1:3:0.1, the solvent is toluene, and the amount of solvent is 2-methyl -20 times the amount of 2-propyl-3-hydroxypropyl-N-isopropyl carbamate.
[0038] Dissolve 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate in toluene, add urea and activate ZnO, the reaction temperature is 110°C, react until no ammonia is released, Cool down and centrifuge the solid, and save it for the next experiment, add water and NaHCO to the organic phase 3 solution, washed until neutral, removed the water phase, and cooled the organic phase until crystals precipitated to obtain a white or off-white powder with a yield of 82% and a melting point of 92-94°C.
Embodiment 2
[0040] The mass ratio of 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate: urea: activated ZnO is 1:2.5:0.1, the solvent is toluene, the reaction temperature is 110°C, the solvent The amount is 20 times that of 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate.
[0041] Other operations are as in Example 1, and the product yield is 81.5%.
Embodiment 3
[0043] The ratio of 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate: urea: activated ZnO is 1:2.0:0.1, the solvent is toluene, the reaction temperature is 110°C, the solvent The amount is 20 times that of 2-methyl-2-propyl-3-hydroxypropyl-N-isopropyl carbamate.
[0044]Other operations are as in Example 1, and the product yield is 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com