Method for preparing high-purity torasemide and crystal form I thereof
A technology for crystallization of torasemide, which is applied in the field of preparation of high-purity torasemide and its crystal form I, can solve the problems of low product purity and high toxicity, and achieve strong controllability, simple operation, Ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
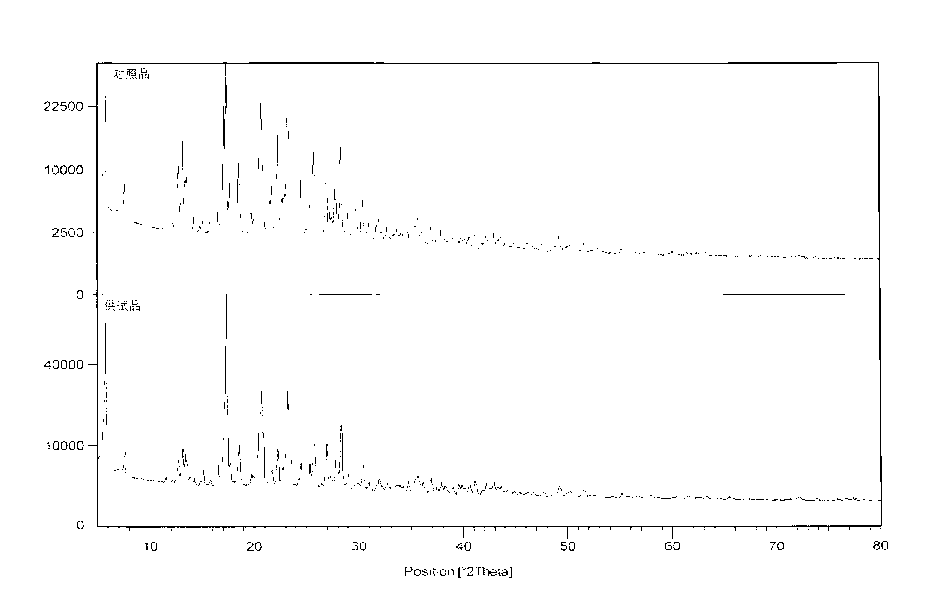
Image
Examples
Embodiment 1
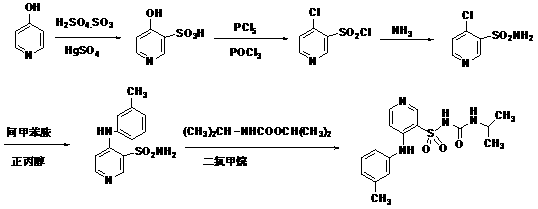
[0038] Example 1 Preparation of crude 3-sulfamoyl-4-(3-methylphenyl)aminopyridine (4, MPSA)
[0039] Add 75 g of 4-chloro-3-pyridinesulfonamide (2), 60 ml of m-methylaniline (3), and 400 ml of isopropanol into the reaction flask, and stir at room temperature until the reaction is complete. Evaporate isopropanol under reduced pressure, add sodium hydroxide solution, stir to dissolve, wash twice with dichloromethane 75ml×2, adjust the water phase to PH=5-8 with concentrated hydrochloric acid at room temperature, stir and filter to obtain MPSA (4) crude product 95g, yield 92.5%.
Embodiment 2
[0040] Example 2 Purification of 3-sulfamoyl-4-(3-methylphenyl)aminopyridine (4, MPSA)
[0041] Add 95 g of MPSA (4) crude product, 700 ml of ethyl acetate, and 2 g of activated carbon to the reaction bottle, heat and reflux for 20 minutes, filter, cool and crystallize the filtrate, filter, and dry the filter cake to obtain 75 g of MPSA refined product, with a yield of 76.5%. Purity (HPLC)>99%.
Embodiment 3
[0042] Example 3 Preparation of Torsemide (5)
[0043] Add MPSA (4) refined product (70g, 0.27mol), CDI (43g, 0.27mol) and 700ml of dichloromethane to the reaction bottle, stir the reaction at 10-40°C, TLC detects that the reaction is complete, add isopropylamine (23ml, 0.27 mol), maintain the temperature and stir the reaction, and TLC detects that the reaction is complete.
[0044]Wash the reaction solution with water, concentrate the organic phase to dryness, add 450ml of 1mol / L sodium hydroxide solution, stir for 30 minutes, filter, add 400ml of ethanol to the filtrate, adjust the pH to 5-6 with acetic acid, stir and crystallize at 0-20°C, and rotate at 120°C ~240 rev / min, filter, and dry the filter cake under reduced pressure at 75-85℃ and vacuum degree of 400~760mmHg to obtain white crystalline particles, 82g, yield: 87.2%, average particle size 100~300μm, HPLC purity: 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com