Novel method for efficiently preparing beta-aminoketone by utilizing dimethyl sulfoxide
A technology of aminoketone and acetophenone, which is applied in the field of organic synthetic chemistry, can solve the problems of high toxicity to the human body, and achieve the effect of overcoming the large toxicity of the environment and the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
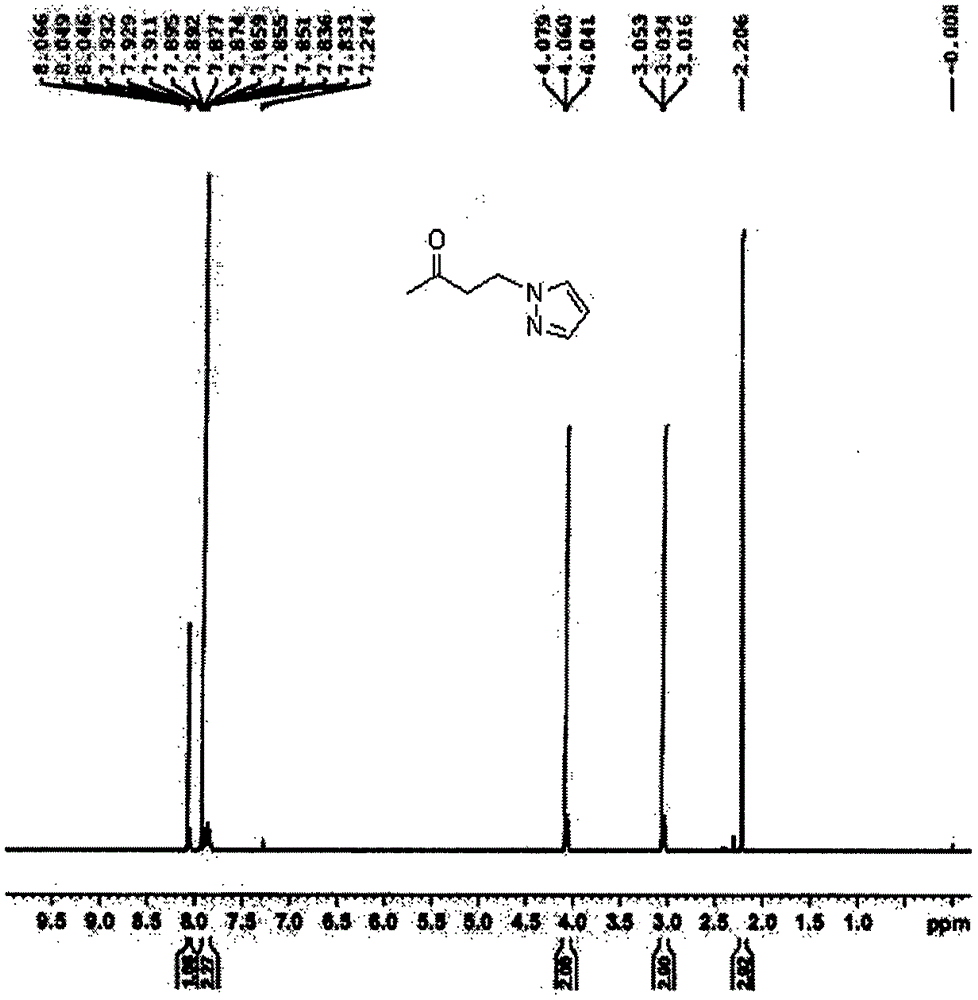
[0020] In a 50 ml round bottom flask, add 0.1202 g (1.0 mmol) of acetophenone, 0.0075 g (1.1 mmol) of imidazole, 0.0172 g (0.1 mmol) of ruthenium trichloride, 0.0198 g of 1,10-phenanthrene Phenyl (0.1 mmol), 0.7080 g (2.0 mmol) selectfluor, 0.2160 g (2.0 mmol) sodium carbonate, 6.0 milliliters of dimethyl sulfoxide (DMSO), heated at 120 ° C, refluxed for 1 hour until the reaction of acetophenone was complete ( Thin layer chromatography TLC monitoring). The reaction mixture was poured into 20 mL of water, extracted with ethyl acetate (10 mL×3). The organic phases were combined and dried over anhydrous sodium sulfate. After distilling off the solvent, the residue was separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=6 / 1) to obtain 0.2866 g of a white solid, with a yield of 91%. The reaction is shown in the following formula:
[0021]
[0022] Spectral analysis data
[0023] White solid.Mp: 138-140℃; 1 H NMR (400MHz; CDCl 3 ): δ=3.59(t,...
Embodiment 2
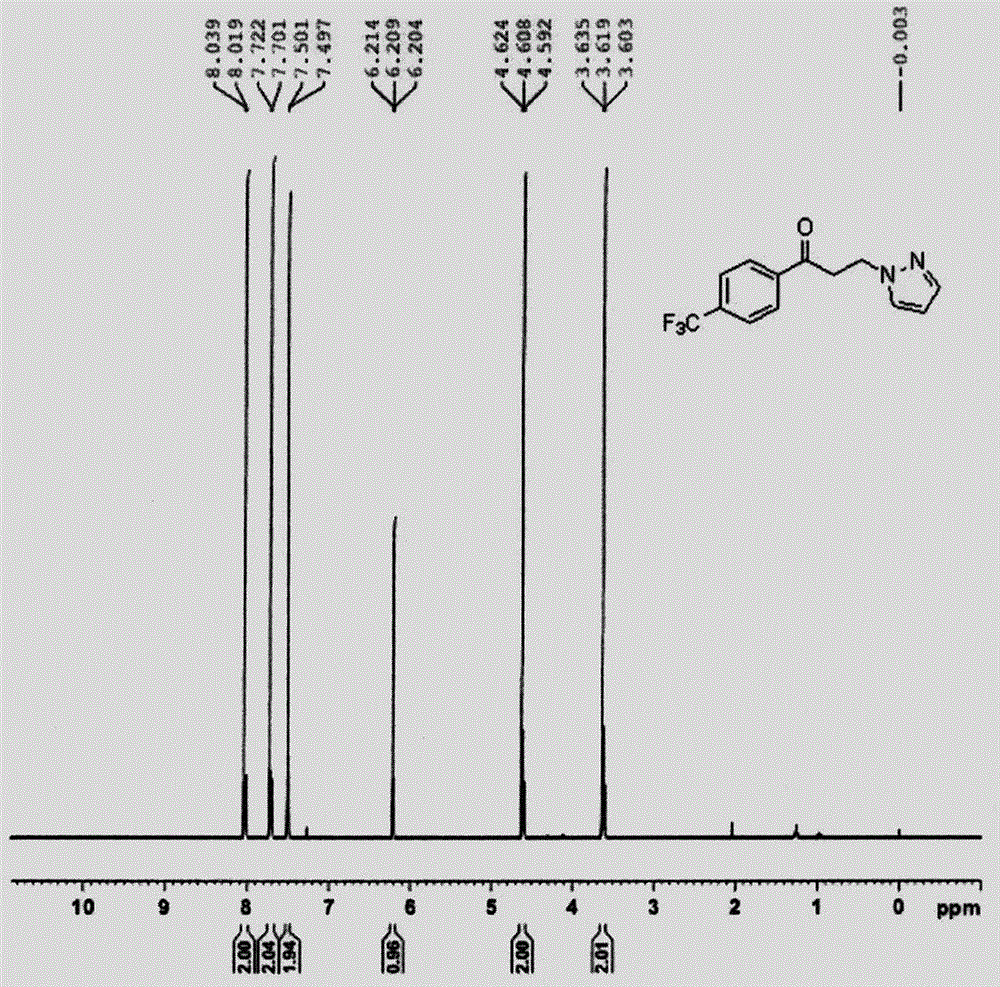
[0025] In a 50 ml round bottom flask, add 0.1682 g (1.0 mmol) 3,5-bis(trifluoromethyl)acetophenone, 0.0075 g (1.1 mmol) imidazole, 0.0172 g (0.1 mmol) trichloride Ruthenium, 0.0198 g 1,10-phenanthroline (0.1 mmol), 0.7080 g (2.0 mmol) selectfluor, 0.2160 g (2.0 mmol) sodium carbonate, 6.0 ml dimethyl sulfoxide (DMSO), heating at 120°C , Refluxed for 1 hour until the reaction of 3,5-bis(trifluoromethyl)acetophenone was complete (monitored by TLC). The reaction mixture was poured into 20 mL of water, extracted with ethyl acetate (10 mL×3). The organic phases were combined and dried over anhydrous sodium sulfate. After distilling off the solvent, the residue was separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=5 / 1) to obtain 0.2994 g of a light yellow solid, with a yield of 81%. The reaction is shown in the following formula:
[0026]
[0027] Spectral analysis data
[0028] White solid.Mp: 129-130℃; 1 H NMR (400MHz; CDCl 3 ): δ=3.63(t...
Embodiment 3
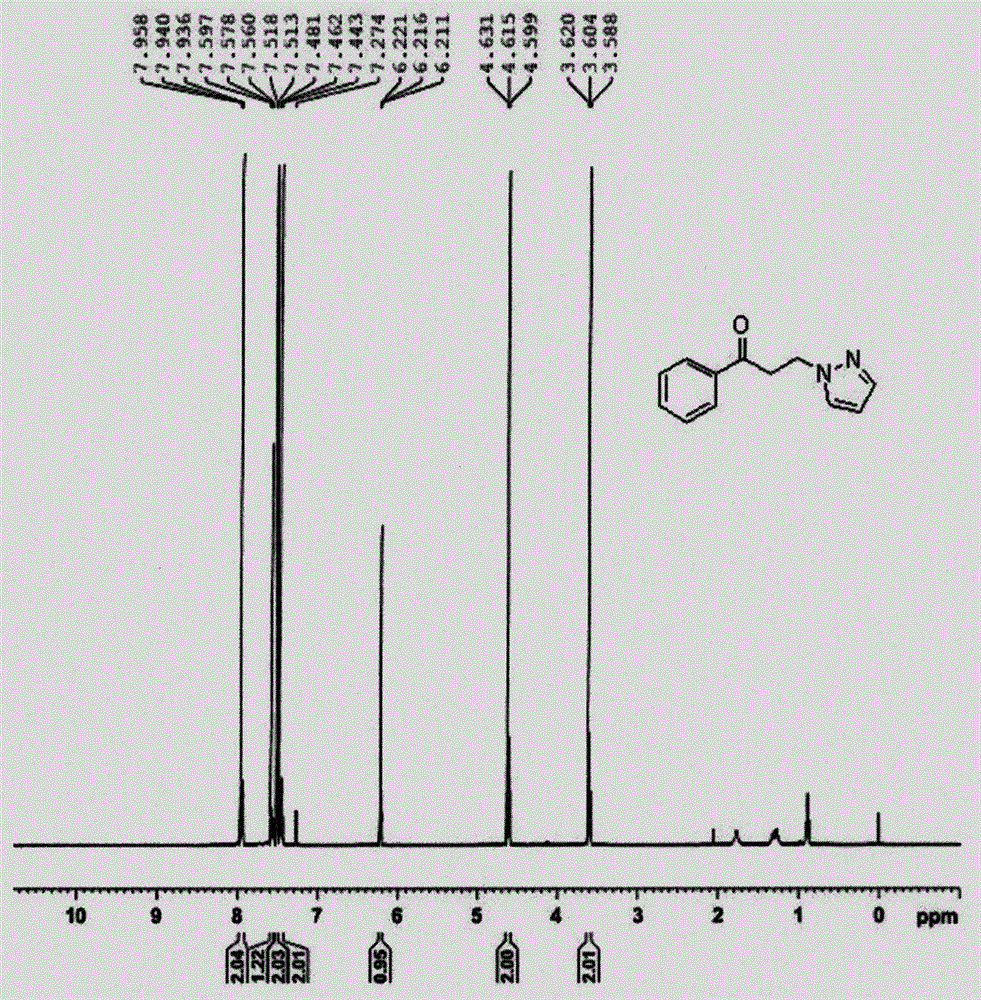
[0030] In a 50 ml round bottom flask, add 0.1702 g (1.0 mmol) of β-naphthyl ethyl ketone, 0.0075 g (1.1 mmol) of imidazole, 0.0172 g (0.1 mmol) of ruthenium trichloride, 0.0198 g (0.1 mmol) 1,10-phenanthroline, 0.7080 g (2.0 mmol) selectfluor, 0.2160 g (2.0 mmol) sodium carbonate, 6.0 ml dimethyl sulfoxide (DMSO), heated at 120 ° C, refluxed for 1 hour to β-naphthyl ethyl The ketone reaction was complete (monitored by TLC). The reaction mixture was poured into 20 mL of water, extracted with ethyl acetate (10 mL×3). The organic phases were combined and dried over anhydrous sodium sulfate. After distilling off the solvent, the residue was separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=5 / 1) to obtain 0.3234 g of a light yellow solid, with a yield of 87%. The reaction is shown in the following formula:
[0031]
[0032] Spectral analysis data
[0033] White solid.Mp: 138-138°C; 1 H NMR (400MHz; CDCl 3 ): δ=3.73(t, J=7.6Hz, 2H), 4.34(t, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com