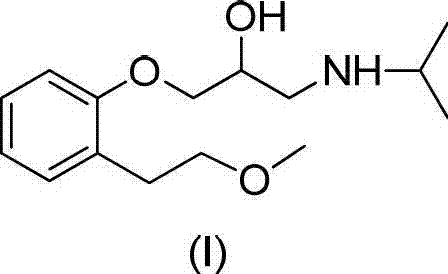
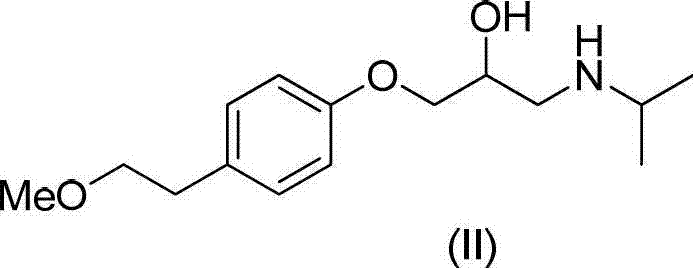
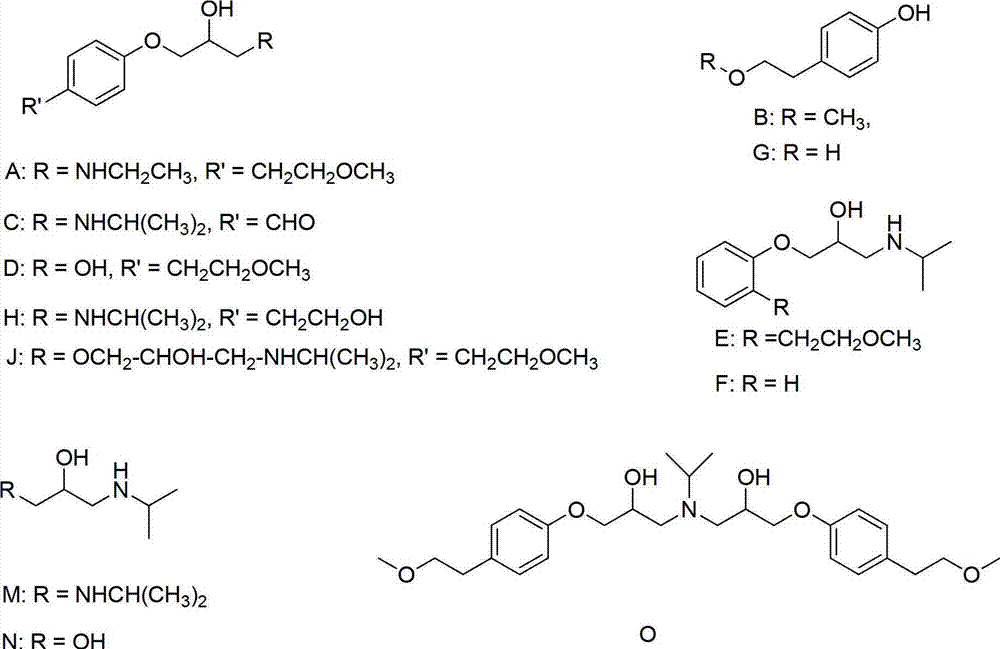
Preparation method of related substance E of metoprolol
A compound, the technology of methoxyethyl, is applied in the field of preparation of metoprolol related substance E, which can solve the problems of low total yield, high price and high cost, and achieve the effect of simple operation, simplified operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The technical scheme of the present invention is further described below with specific examples, but protection scope of the present invention is not limited to this: Embodiment one: the synthesis of 2-hydroxyphenethyl alcohol (IV)
[0054] Add 30.4g of compound (Ⅲ), 300mL of tetrahydrofuran and 24.2g of triethylamine into the reaction flask, control the temperature at 0-10°C, add 20.8g of methyl chloroformate dropwise to the reaction system, and react at room temperature for 1h after the dropwise addition. Filter, cool the filtrate to 0~10°C, add 11.4g of sodium borohydride in 100mL aqueous solution to the filtrate. After the dropwise addition, it was raised to room temperature and reacted for 3 hours, and the reaction was completed. Add 300 mL of ethyl acetate and 300 mL of saturated brine for extraction, and separate the organic layer. The organic layer was dried and concentrated to obtain 30.5 g of oil, which was directly carried out to the next reaction.
Embodiment 2
[0055] Embodiment two: the synthesis of 2-benzyloxyphenethyl alcohol (Ⅴ)
[0056] Add the compound (IV) prepared in Example 1, 56.7g of benzyl bromide, 91.5g of potassium carbonate and 300mL of acetone into the reaction flask, heat up to reflux for 20 hours, and the reaction is complete. Filter and wash the filter cake with acetone. After the filtrate was concentrated, 300 mL of ethyl acetate and 300 mL of water were added for extraction, and the organic layer was separated. The organic layer was dried, and the solvent was removed under reduced pressure to obtain 48.9 g of oil, which was directly put into the next reaction.
Embodiment 3
[0057] Example three: Synthesis of 1-benzyloxy-2-(2-methoxyethyl)benzene (Ⅵ)
[0058] Add 12.3g of 60% sodium hydride and 300mL of N,N-dimethylformamide to the reaction flask, lower the temperature to 0~10°C, and add dropwise 50mL of N,N-dimethylformamide of compound (Ⅴ) prepared in Example 2 solution. After dropping, rise to room temperature and react for 2h. Add 45.6g of methyl iodide dropwise to the system, then react at room temperature for 20h, and the reaction is complete. Add 300 mL of saturated brine dropwise to the system, add 300 mL of ethyl acetate for extraction, and separate the organic layer. The aqueous layer was extracted once more with ethyl acetate, and the organic layers were combined. The organic phase was dried, and the solvent was concentrated to obtain 51.7 g of oil, which was directly put into the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com