Method for preparing l-betaxolol hydrochloride
A technology of betaxolol hydrochloride and molar ratio, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of amino hydroxyl compounds, can solve the problems of high cost, high energy consumption, and difficult product separation, and achieve easy separation , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
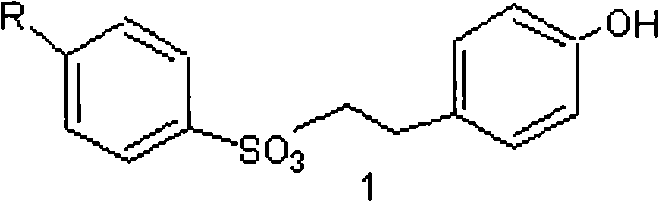
[0045] a) Preparation of compound 1 1-p-toluenesulfonyl-2-p-hydroxyphenylethane.
[0046] According to the method described in literature synthesis 2003, 4, 509-512, 1-p-toluenesulfonyl-2-p-hydroxyphenylethane was prepared.
[0047] Dissolve p-hydroxyphenethyl alcohol (6.90g, 50.0mmol) in 10.0ml of pyridine, then add a mixture of p-toluenesulfonyl chloride (10.03g, 52.5mmol) and 10.0ml of pyridine, and stir at -10°C for 25min. Stirred at 0°C for 2h, and reacted at 10°C for 12h. After the reaction, add 125ml of ice water and stir for 1h, then extract with diethyl ether (2×75ml), wash the ether layer with 1% HCl (2×50ml), wash with anhydrous Na 2 SO 4 After drying, filtration and concentration, 14.6 g of compound 1, 1-p-toluenesulfonyl-2-p-hydroxyphenylethane was obtained, with a yield of 95%.
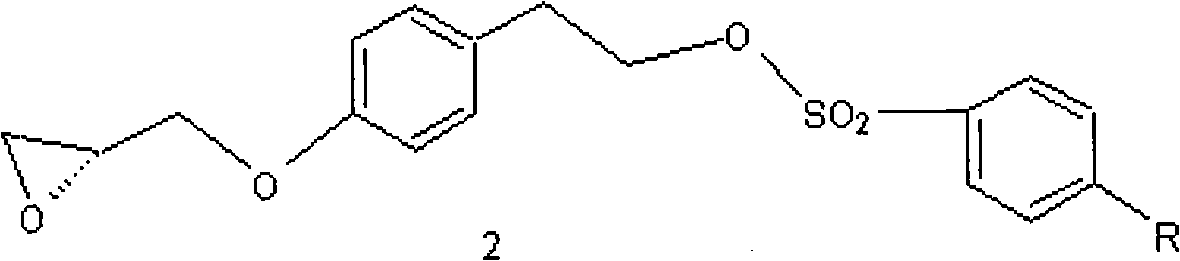
[0048] b) Preparation of compound 2(2S)-3-(4-p-toluenesulfonyloxyethylphenoxy)-1,2-propylene oxide.
[0049] Compound 1 (29.2g, 0.1mol) was dissolved in 50ml of ethanol, and then 11....
Embodiment 2
[0058] Embodiment 2 prepares compound 2
[0059] Compound 1 (29.2g, 0.1mol) and 40 grams of 20% NaOH solution were dissolved in 120ml of water at 5-15°C, then 11.3 grams of R-epichlorohydrin and 3 grams of benzyltriethylammonium chloride were added, and then The reaction was carried out at this temperature for 35 hours. Use TLC (developing agent ethyl acetate: methanol = 2: 1) to detect, after the reaction is completed, filter, after the filtrate is concentrated under reduced pressure (temperature 30-35 ° C, pressure 5-20mmHg) to remove the solvent, add 120ml of dichloromethane to dissolve , washed with 50ml of water, the organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure (temperature 20-50°C, pressure 20-100mmHg) to remove the solvent to obtain intermediate 2, (2S)-3-(4- 33.5 grams of p-toluenesulfonyloxyethylphenoxy)-1,2-propylene oxide, the yield is 86.2%,
Embodiment 3
[0060] Embodiment 3 prepares compound 2
[0061] Compound 1 (29.2g, 0.1mol) was dissolved in 50ml of acetone, and then 11.3 grams of R-epichlorohydrin and 20 grams of anhydrous K 2 CO 3 , React at 30-35°C for 25 hours. Use TLC (developing agent ethyl acetate: methanol = 2: 1) to detect, after the reaction is completed, filter, after the filtrate is concentrated under reduced pressure (temperature 30-35 ° C, pressure 5-20mmHg) to remove the solvent, add 120ml of dichloromethane to dissolve , washed with 50ml of water, the organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure (temperature 20-50°C, pressure 20-100mmHg) to remove the solvent to obtain intermediate 2, (2S)-3-(4- 27.2 g of p-toluenesulfonyloxyethylphenoxy)-1,2-propylene oxide, the yield was 78.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com