Amino evodiamine polymer micelle as well as preparation method and application thereof
A technology of aminoevodiamine and polymer glue, which can be applied in the direction of drug combination, drug delivery, and pharmaceutical formulation, and can solve the problems of anti-tumor activity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0033] See another application submitted by the applicant on the same day, the invention title is aminoevodiamine derivatives and their preparation method and application, which discloses aminoevodiamine.
[0034] First synthesize 2 compounds with nitro groups, 2-nitroevodiamine (7a) and 10-methoxy-2-nitroevodiamine (7b); then generate 2-aminoevodiamine (8a) through reduction reaction and 10-methoxy-2-aminoevodiamine (8b). The synthetic route is as follows:
[0035]
[0036] Synthesis of Compound 3,4-Dihydro-β-carboline (3a)
[0037]Add 4g of tryptamine (1a) (M=160.22, 24.97mmol) and 120mL of ethyl formate (both reactant and solvent) into a 250mL one-necked flask to obtain a clear light yellow liquid, heat to reflux at 60°C for 18 hours. Rotary steaming, an oily liquid was obtained. Add dichloromethane to the flask until it dissolves. Under the condition of 0-5°C ice-salt bath, use the dropping funnel to drop (10 minutes to finish) 10ml POCl 3 , reacted for 2h under ice...
Embodiment 1
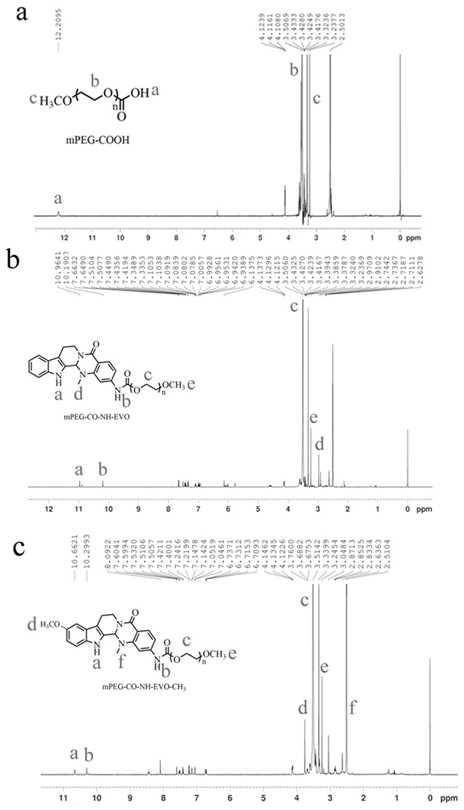
[0043] Example 1 Synthesis of aminoevodiamine polymer conjugates
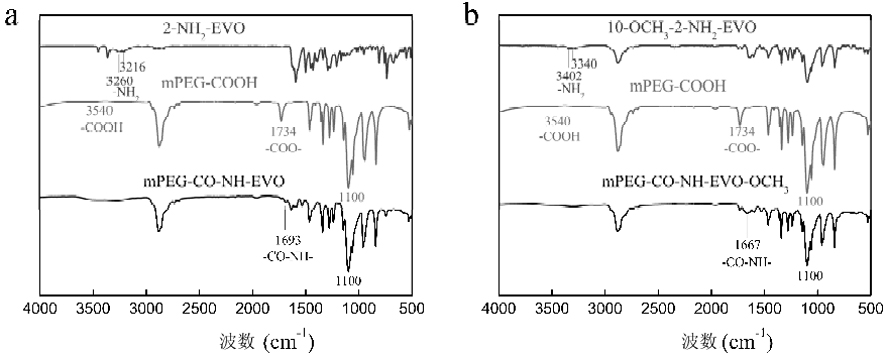
[0044] Carboxy- and methoxy-terminated polyethylene glycol (mPEG-COOH 2000 ) respectively with the aminoevodiamine derivative 2-NH 2 -EVO, 10-OCH 3 -2-NH 2 -EVO reaction, under the protection of nitrogen, use DMAP as catalyst and EDC as dehydrating agent to generate mPEG-CO-NH-EVO and mPEG-CO-NH-EVO-OCH 3 Conjugates. The synthetic route is as follows:
[0045]
[0046] 122.38 mg (0.061 mmol) mPEG-COOH 2000 Dissolved in 20 mL of dry DCM, 39.73 mg (0.125 mmol) of 2-NH 2 - Dissolve EVO in 3mL DMF, add 17mL dry DCM, and stir evenly with magnetic force. Under nitrogen protection, 10 mL of DCM solution containing 100 mg (0.522 mmol) of EDC and 8 mg (0.066 mmol) of DMAP was slowly dripped with a syringe under the condition of 0 °C ice bath, reacted at 0 °C for 2 h, and then reacted at room temperature for 48 h. After the reaction was complete, wash with saturated sodium carbonate solution, then dilute hydro...
Embodiment 2
[0053] The preparation of embodiment two polymer micelles
[0054] Prepare amphiphilic polymer micelles by solvent evaporation method, weigh 10 mg of mPEG-CO-NH-EVO conjugate, dissolve in 1 mL of tetrahydrofuran, drop into stirred 10 mL of ultrapure water, and continue stirring for 30 min. Then let it stand in a fume hood for 20 hours, and volatilize the dissolved tetrahydrofuran at room temperature, thereby preparing the amphiphilic polymer micelles mPEG-CO-NH-EVO micelles, which are located in water, and after the water volatilizes, a pure gum is obtained. bundle.
[0055] mPEG-CO-NH-EVO-OCH 3 The preparation of micelles was the same as above.
[0056] As a contrast, replace the 2-NH of the present invention with existing aminoevodiamine derivatives 2 -EVO, through the same method, mPEG-CO-NH-ENH micelles are obtained; the chemical structural formula of the existing aminoevodiamine derivatives is as follows:
[0057]
[0058] Characterization of polymer micelles
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com