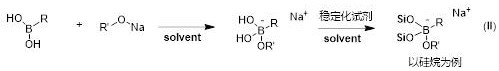
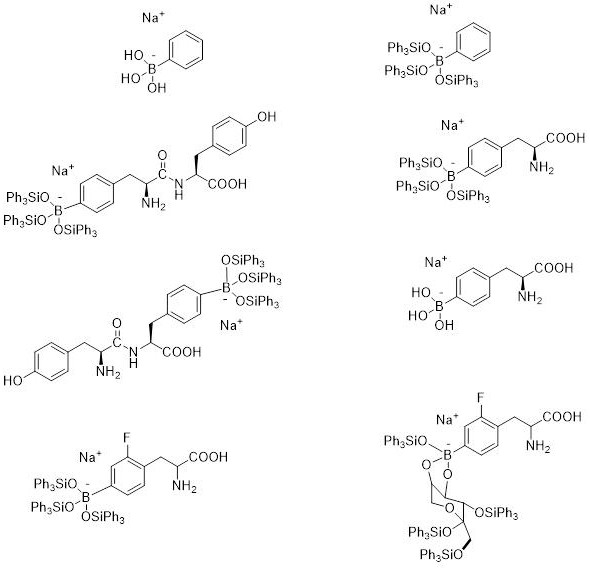
Borate compound for cancer boron neutron capture therapeutic drug and preparation thereof
A boron neutron capture, boric acid compound technology, applied in the field of medicinal chemistry and radiation medicine, can solve the problems of limited research, high instability, etc., to achieve the effect of expanding the scope, strong selectivity, and good efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of borate compound (1) (two-step method).
[0057]
[0058] Under air atmosphere, (L)-4-dihydroxyboryl phenylalanine (0.05 mmol, 10.45 mg), sodium hydroxide (0.15 mmol, 6 mg ), toluene (4 mL) and a magnetic stir bar were added to 35 mL glass pressure-resistant tube. Then triphenylsilane (0.15 mmol, 39 mg) was added, and the reaction tube was reacted at 90°C for 6 hours. After the reaction is completed, the reaction system is cooled to room temperature, the reaction vessel is opened at room temperature, and is filtered; the obtained solid is recrystallized and separated to obtain the reaction product. The pure product yield of target product is 78%.
[0059] 1 H NMR (300 MHz, DMSO) δ 12.89 (s, 1H), 8.71 (d, 2H), 7.75 (d, 2H), 7.46 (m, 18H), 7.39-7.35 (m, 27H), 7.2(d, 2H), 4.18(s, 1H), 3.42(d, 2H).
[0060] 13 C NMR (100 MHz, DMSO) δ 174.7, 138.3, 136.6, 135.7, 133.3, 132.5, 130.0, 129.5, 127.7, 56.7, 37.3.
Embodiment 2
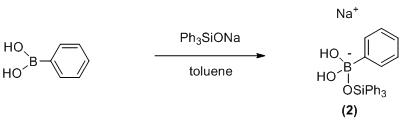
[0061] Embodiment 2: the preparation (one-step method) of borate compound (2)
[0062]
[0063] Under air atmosphere, phenylboronic acid (0.05 mmol, 6.1 mg), sodium triphenylsilanolate (0.025 mmol, 7.46 mg ), and a magnetic stir bar were added to a 35 mL glass pressure-resistant tube. Then toluene (4 mL) was added, and the reaction tube was reacted at 90°C for 6 hours. After the reaction is completed, the reaction system is cooled to room temperature, the reaction vessel is opened at room temperature, and is filtered; the obtained solid is recrystallized and separated to obtain the reaction product. The pure product yield of target product is 75%.
[0064] 1 H NMR (300 MHz, DMSO) δ 7.75(d, 2H), 7.48 - 7.44 (m, 6H), 7.37-7.39(m, 10m) 7.36(s, 1H), 7.35(s, 1H), 4.2 (d , 2H).
[0065] 13 C NMR (101 MHz, DMSO) δ 138.5, 138.3, 133.4, 132.5, 130.0, 129.5, 128.7.
Embodiment 3
[0066] Embodiment 3: the preparation of borate compound (3) (two-step method)
[0067]
[0068] Under air atmosphere, phenylboronic acid (0.05 mmol, 6.1 mg), sodium hydroxide (0.5 mmol, 20 mg), toluene (4 mL) and a magnetic stir bar were added to a 35 mL glass pressure-resistant tube. Then triphenylsilane (0.15 mmol, 39 mg) was added, and the reaction tube was reacted at 90°C for 12 hours. After the reaction is completed, the reaction system is cooled to room temperature, the reaction vessel is opened at room temperature, and is filtered; the obtained solid is recrystallized and separated to obtain the reaction product. The pure product yield of target product is 75%.
[0069] 1 H NMR (300 MHz, DMSO) δ 7.75 (d, 2H), 7.48- 7.44 (m, 18H), 7.34 -7.38 (m, 30H).
[0070] 13 C NMR (101 MHz, DMSO) δ 138.3, 133.4, 132.5, 130.0, 129.5, 128.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com