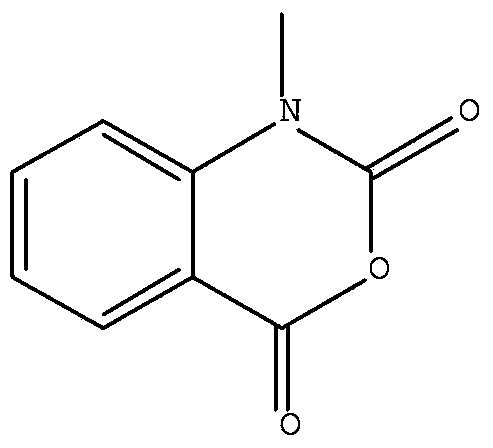
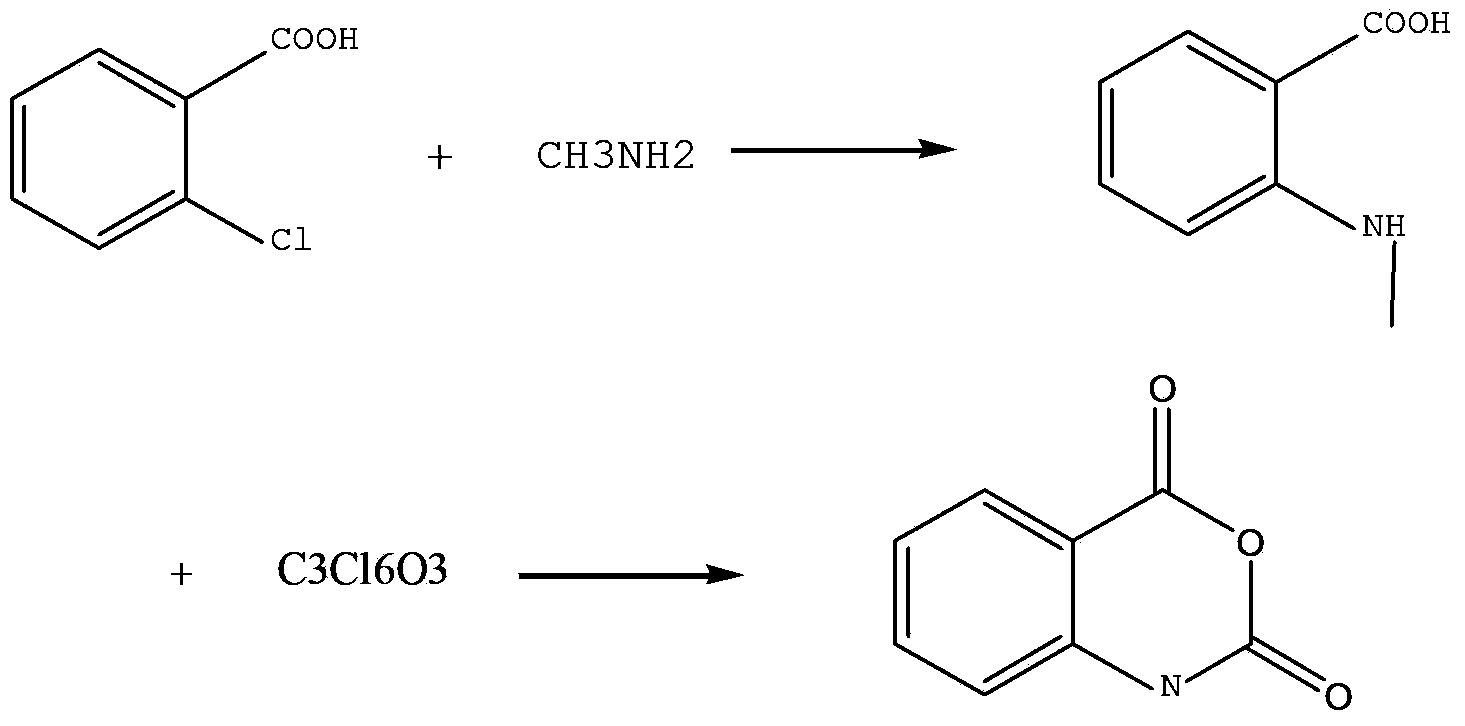
Method for preparing N-methyl isatoic anhydride
A technology of methyl isatoic anhydride and methyl anthranilic acid is applied in the field of preparation of N-methyl isatoic anhydride, and can solve the problems of low yield, difficult availability of raw material N-methyl anthranilic acid and the like , to achieve the effect of short reaction time, cheap and easy-to-obtain raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1, the preparation of N-methyl anthranilic acid
[0029] Feed 62.4g of o-chlorobenzoic acid, 105g of methylamine solution, 28g of anhydrous potassium carbonate, 3g of copper powder, and 40ml of DMF, stir evenly, heat up to 110°C, react for 1h, and stop the reaction. Cool to room temperature, add 100ml of 1:1 glacial acetic acid aqueous solution, and let stand for 2h. Filter and wash with water to obtain 58 g of white solid, which is N-methylanthranilic acid. The yield was 87.8%.
[0030] 2. Preparation of N-methylisatoic anhydride
[0031] Feed 15g of N-methylanthranilic acid and 60ml of dichloromethane, cool down at 0-5°C, slowly add 14g of triphosgene, raise the temperature to 20°C, and react for 2h. Stop the reaction, add 100ml of water, separate the organic phase, wash with water until neutral, recover the solvent under reduced pressure to obtain a white solid, and refine it with ethanol to obtain 15.4g of fine product. The yield was 96%.
Embodiment 2
[0033] 1, the preparation of N-methyl anthranilic acid
[0034] Feed 62.4g of o-chlorobenzoic acid, 145g of methylamine solution, 36.4g of anhydrous potassium carbonate, 3.2g of copper powder, and 50ml of water, stir well, heat up to 80°C, react for 45min, and stop the reaction. Cool to room temperature, add 120ml of 1:1 glacial acetic acid aqueous solution, and let stand for 3h. Filter and wash with water to obtain 52 g of white solid, which is N-methylanthranilic acid. The yield was 78.9%.
[0035] 2. Preparation of N-methylisatoic anhydride
[0036] Feed 15g of N-methylanthranilic acid and 60ml of dichloromethane, cool down at 0-5°C, slowly add 12.5g of triphosgene, warm up to room temperature, and react for 2h. Stop the reaction, add 100ml of water, separate the organic phase, wash with water until neutral, recover the solvent under reduced pressure to obtain a white solid, and refine it with ethanol to obtain 15.5g of fine product. The yield was 97%.
Embodiment 3
[0038] 1, the preparation of N-methyl anthranilic acid
[0039] Feed 62.4g of o-chlorobenzoic acid, 108g of methylamine solution, 30.84g of anhydrous potassium carbonate, 3g of copper powder, and 50ml of water, stir well, heat up to 80°C, react for 30min, and stop the reaction. Cool to room temperature, add 120ml of 1:1 glacial acetic acid aqueous solution, and let stand for 2h. Filter and wash with water to obtain 49 g of white solid. The yield was 74%.
[0040] 2. Preparation of N-methylisatoic anhydride
[0041] Feed 15g of N-methylanthranilic acid and 60ml of toluene, cool down at 0-5°C, slowly add 17.5g of triphosgene, raise the temperature to room temperature at°C, and react for 3.5h. Stop the reaction, add 100ml of water, separate the organic phase, wash with water until neutral, recover the solvent under reduced pressure to obtain a white solid, and refine it with ethanol to obtain the fine product 15.2. The yield is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com