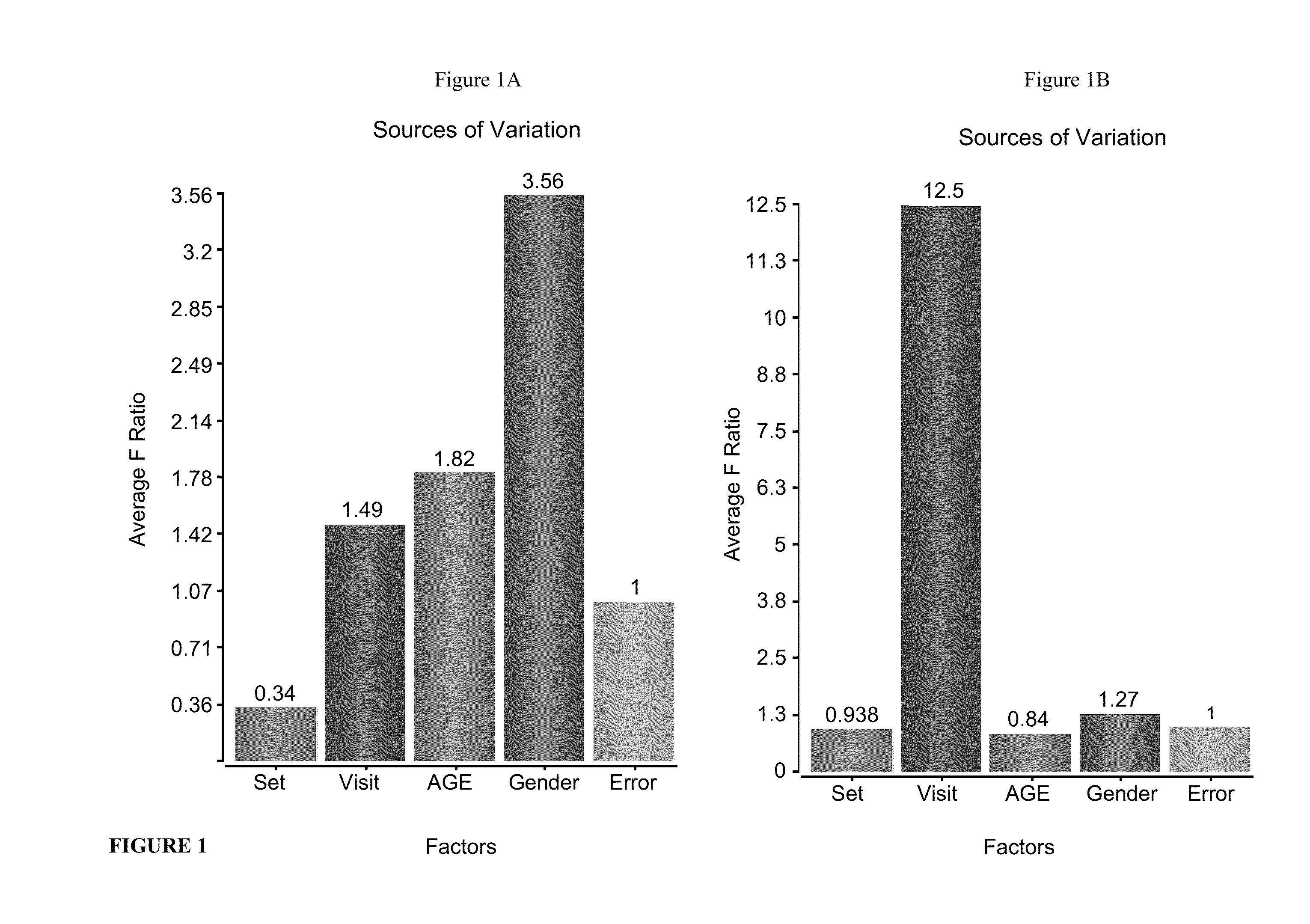
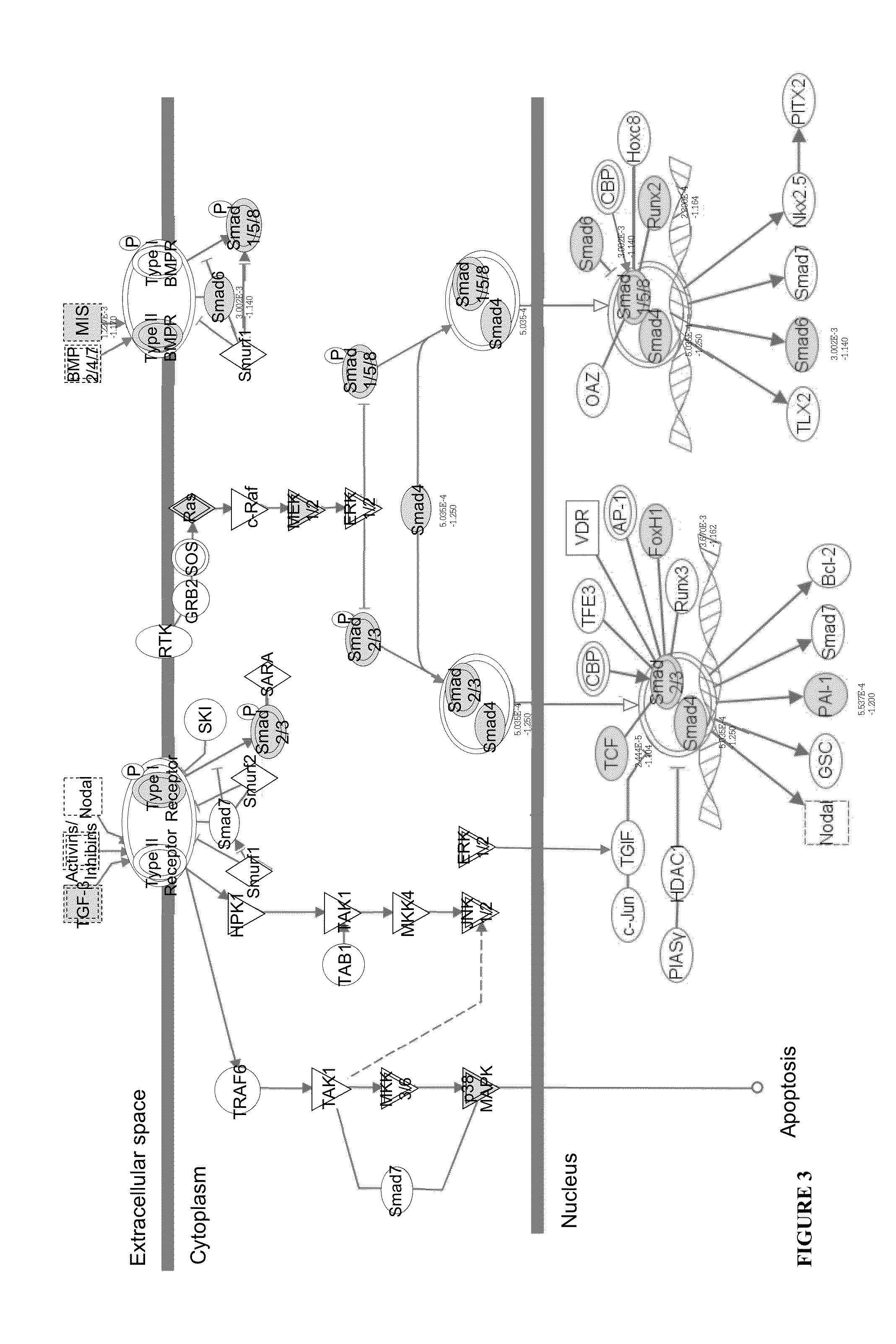
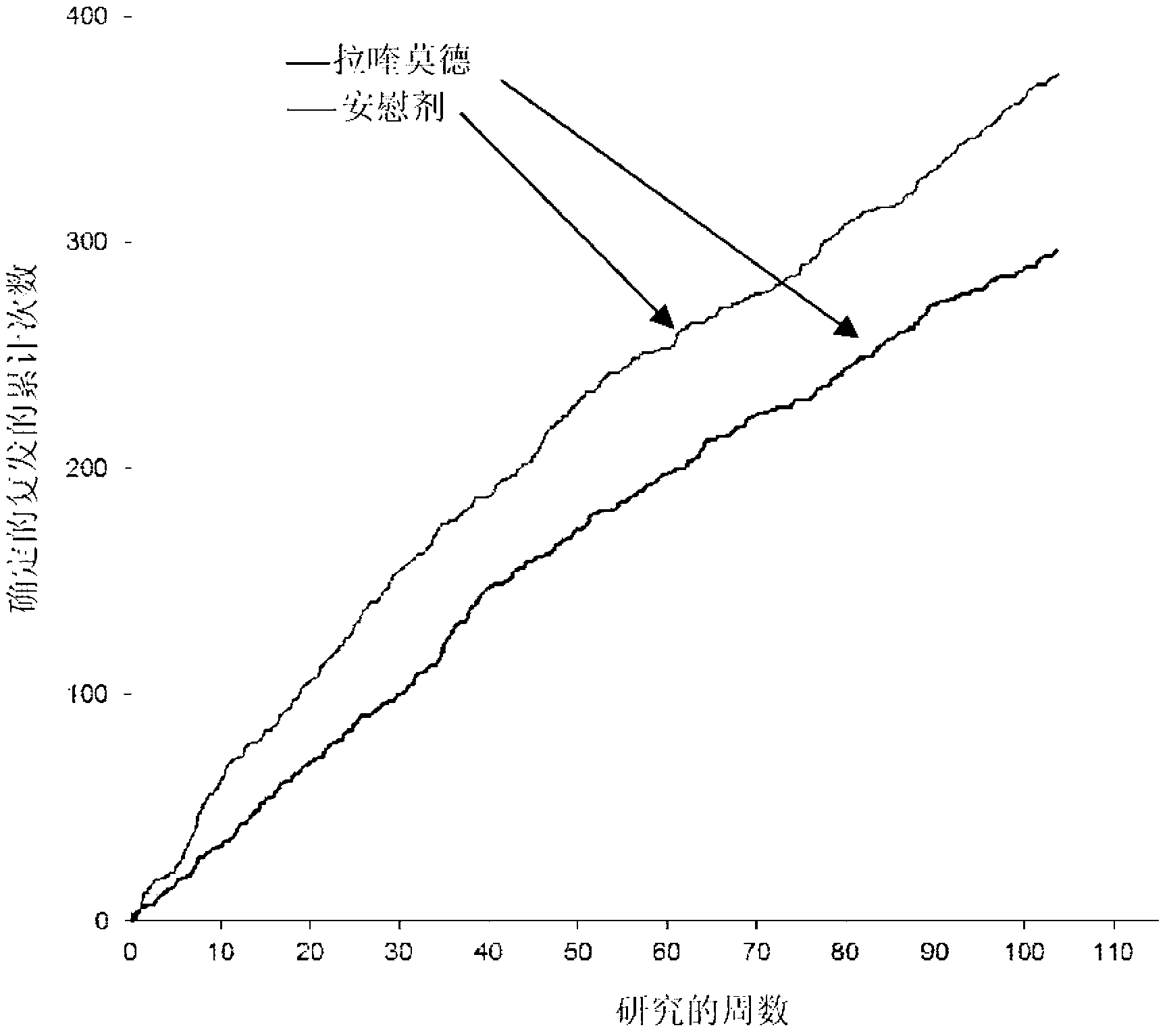
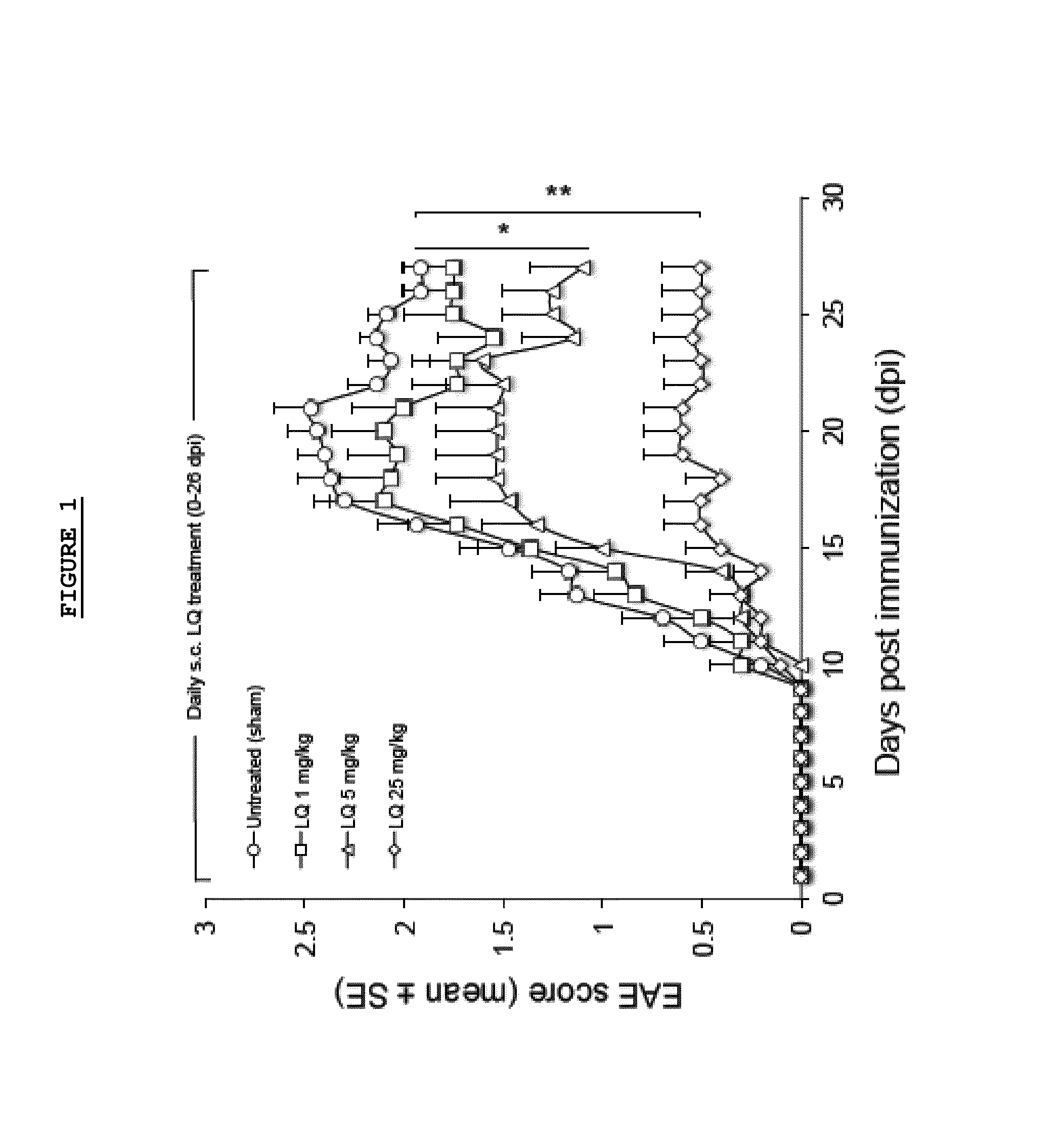
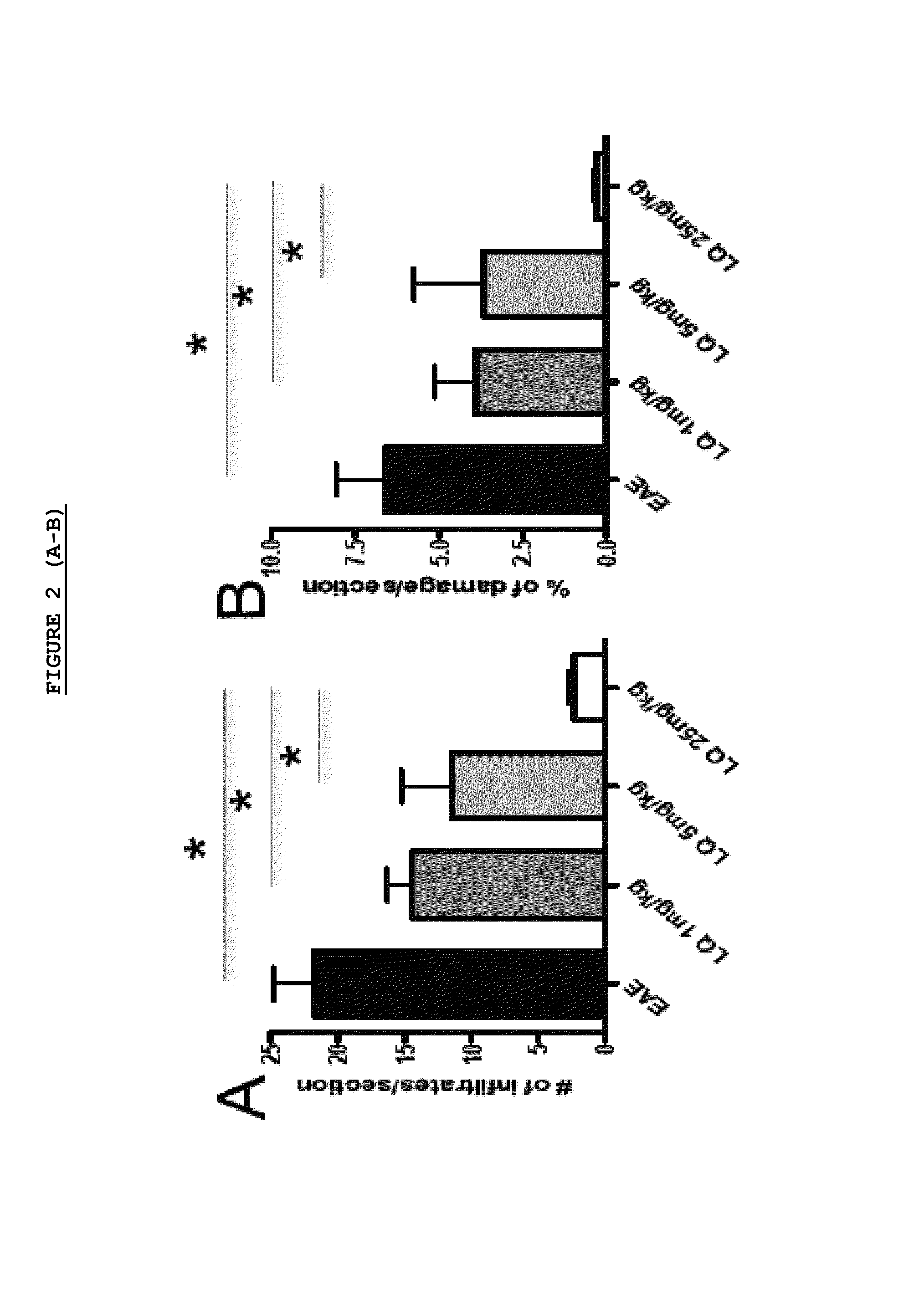
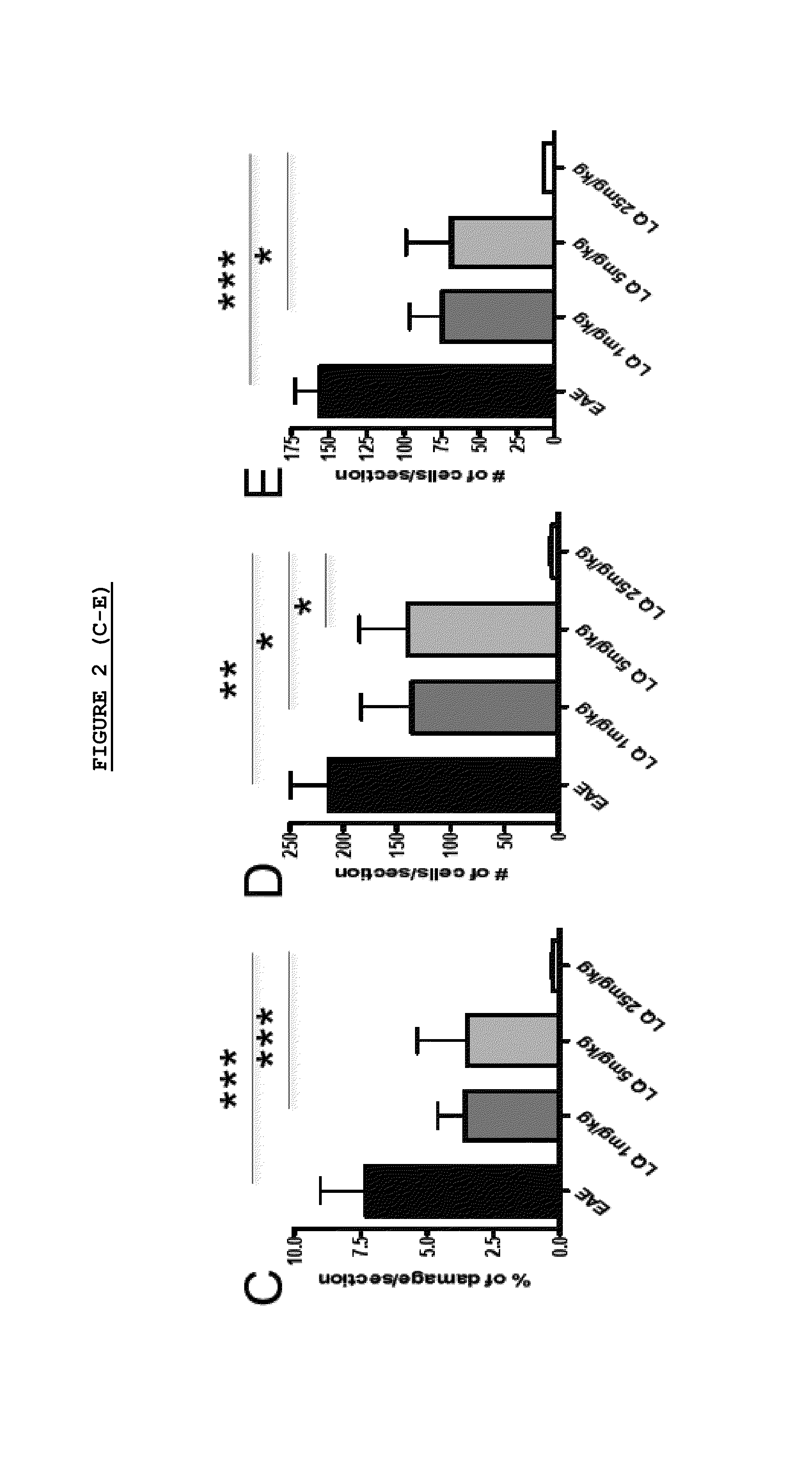
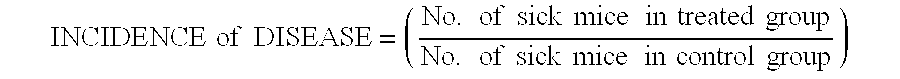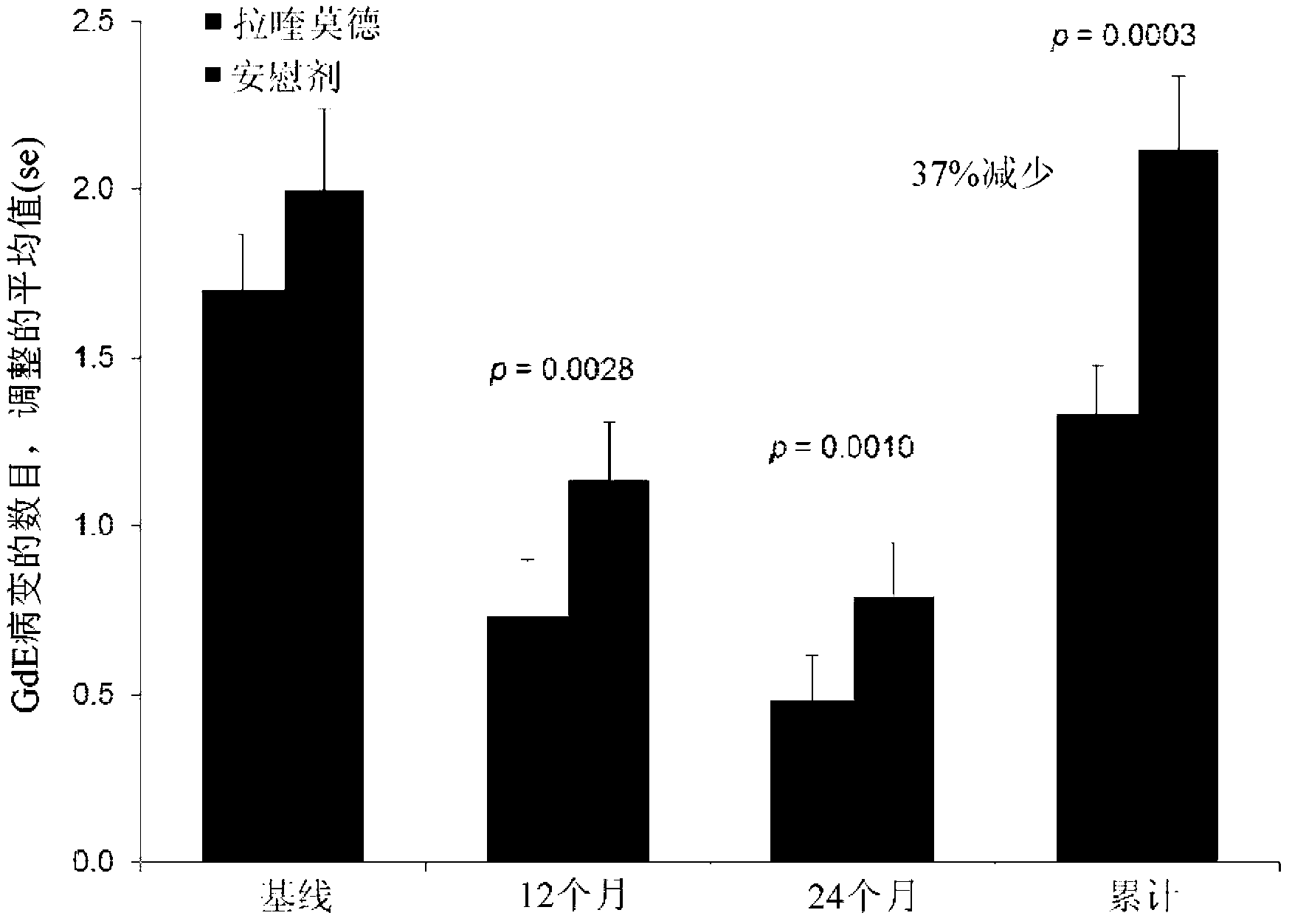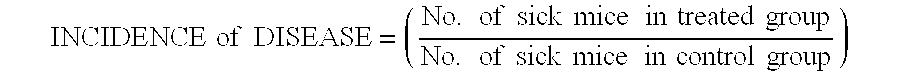Patents
Literature
72 results about "Laquinimod" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
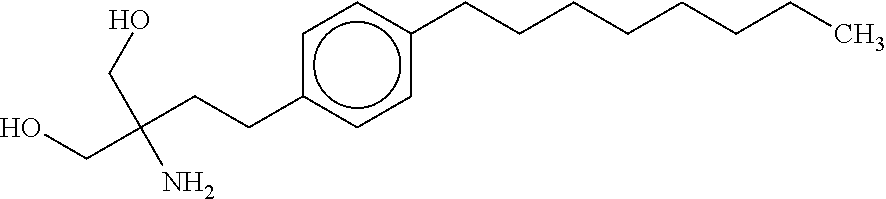
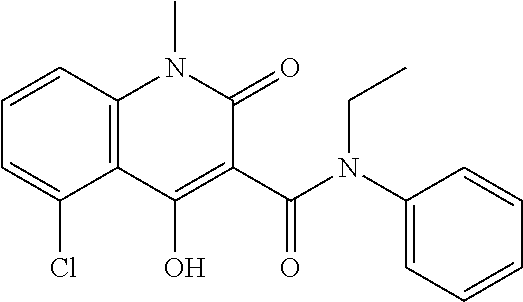
Laquinimod is an experimental immunomodulator developed by Active Biotech and Teva. It is being investigated as an oral treatment for multiple sclerosis (MS). Laquinimod is the successor of Active Biotech's failed experimental immunomodulator linomide.
Treatment of Crohn's disease with laquinimod
This application provides for a method of treating a subject suffering from Crohn's disease, the method comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof effective to treat the subject. This application provides for use of laquinimod in the manufacture of a medicament for treating a subject suffering from Crohn's disease. This application also provides for a pharmaceutical composition comprising laquinimod for use in treating a subject suffering from Crohn's disease.
Owner:ACTIVE BIOTECH AB
Treatment of Crohn's disease with laquinimod
This application provides for a method of treating a subject suffering from Crohn's disease, the method comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof effective to treat the subject. This application provides for use of laquinimod in the manufacture of a medicament for treating a subject suffering from Crohn's disease. This application also provides for a pharmaceutical composition comprising laquinimod for use in treating a subject suffering from Crohn's disease.
Owner:ACTIVE BIOTECH AB
Treatment of BDNF-related disorders using laquinimod
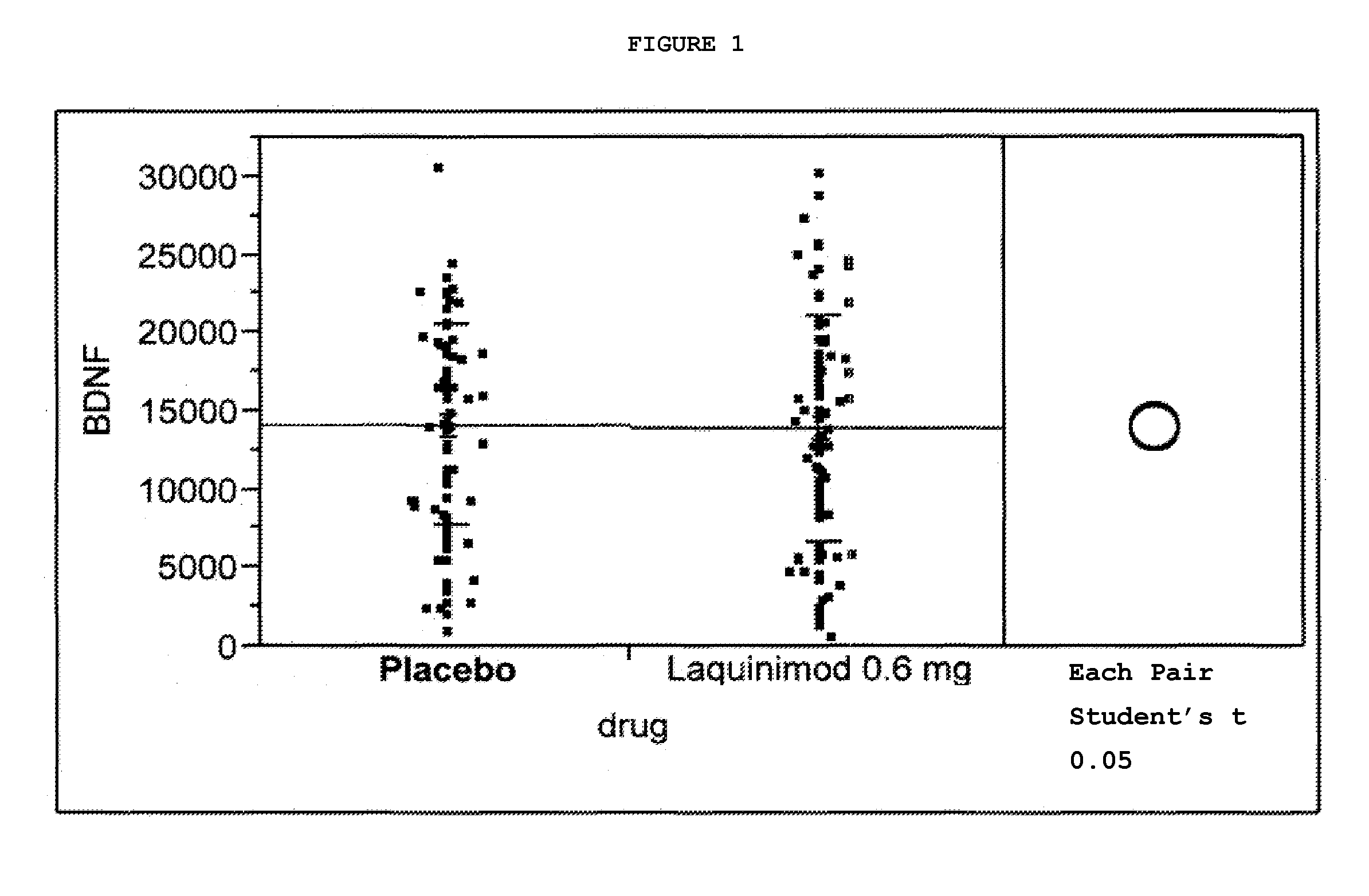
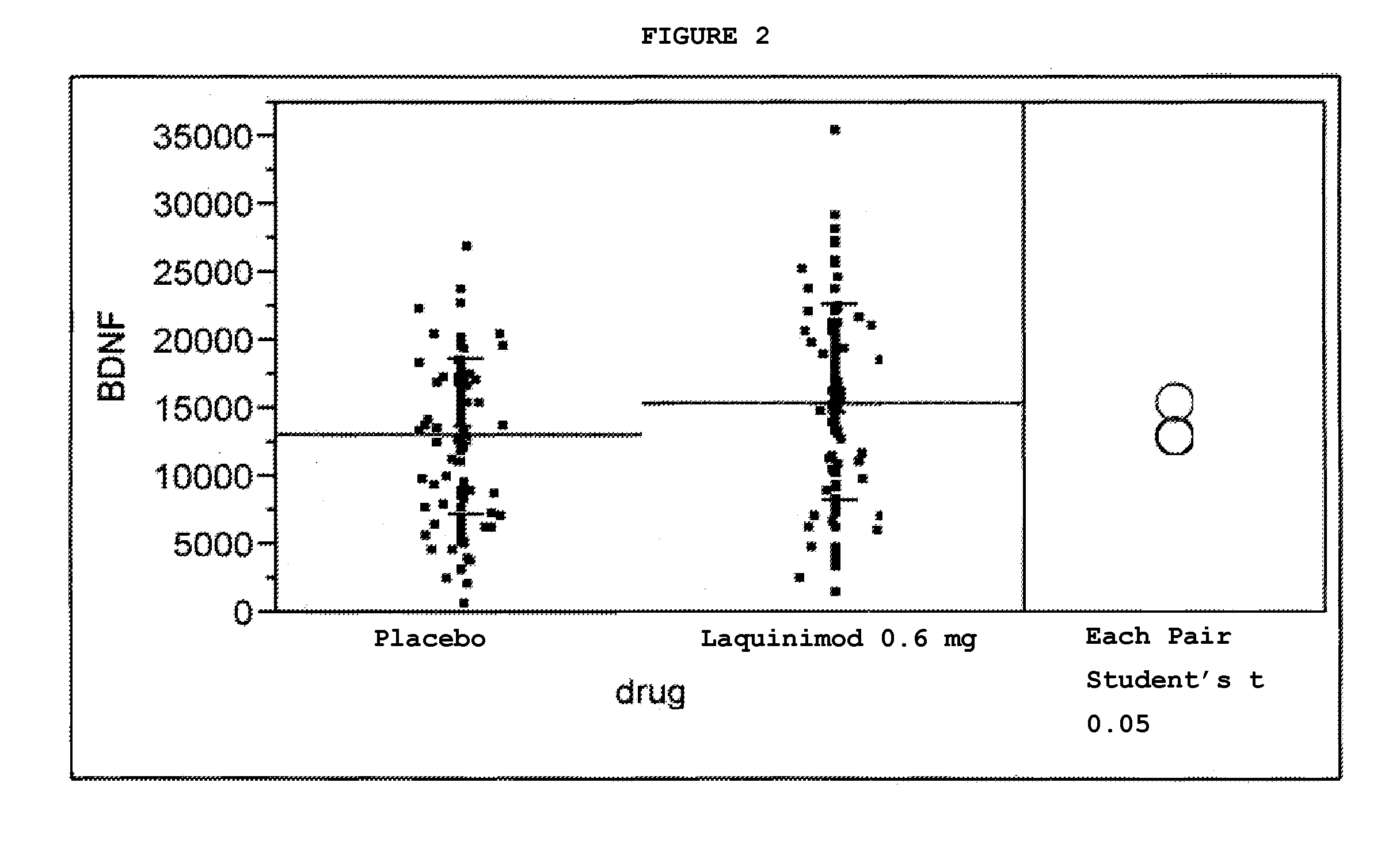
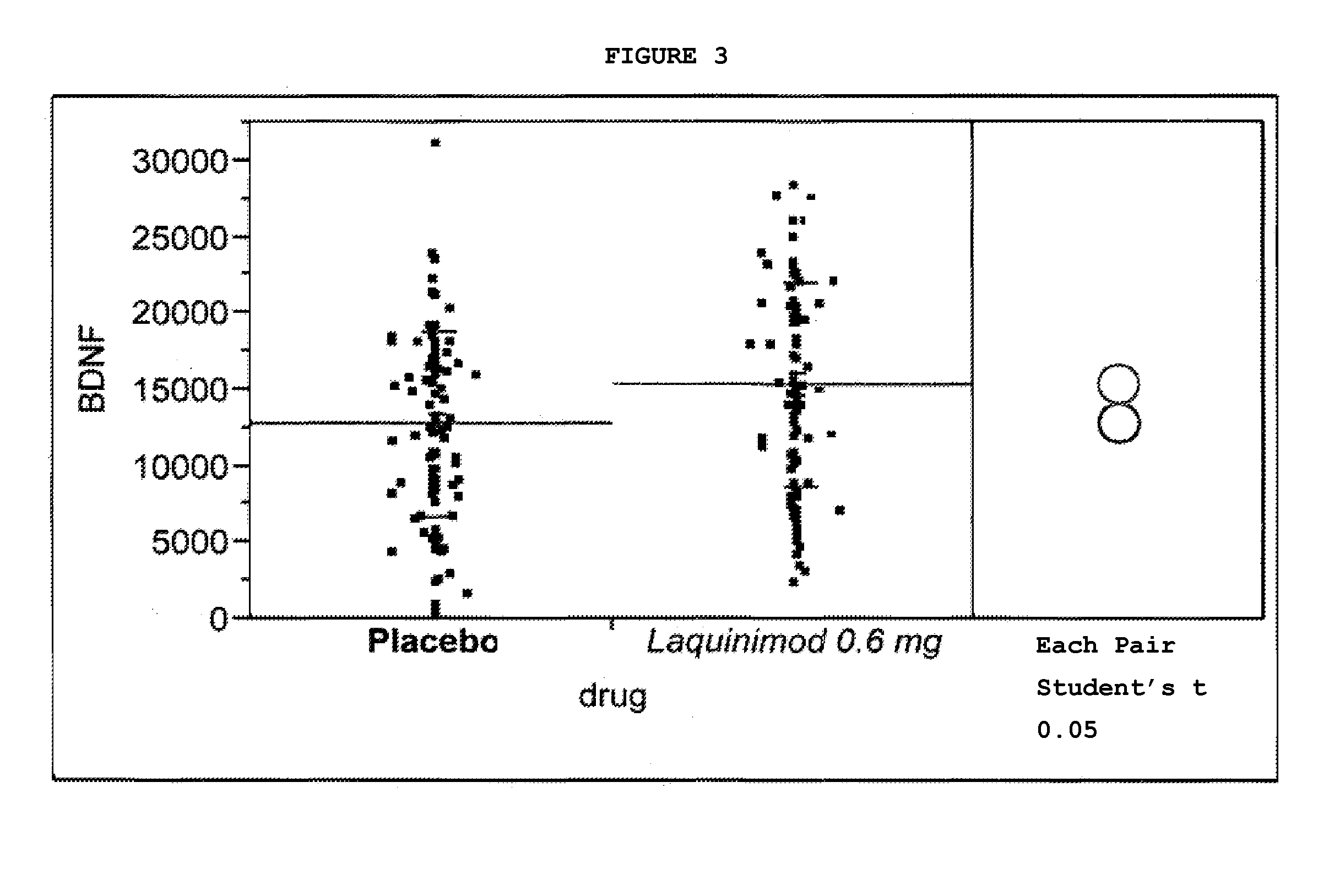
This application provides for a method of increasing brain-derived neurotrophic factor (BDNF) serum level in a human subject comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof effective to increase BDNF serum level in the human subject. The method can further comprise periodically administering to the subject an amount of a second BDNF-increasing agent. This application also provides for a method for treating a human subject suffering from a BDNF-related disease comprising periodically administering laquinimod or a pharmaceutically acceptable salt thereof in an amount effective to treat the human subject. This application additionally provides for use of laquinimod in the manufacture of a medicament for increasing BDNF serum level in a human subject. This application further provides for a pharmaceutical composition comprising an amount of laquinimod effective for use in increasing BDNF serum level in a human subject. This application also provides for a pharmaceutical preparation comprising an amount of laquinimod and an amount of a second BDNF-increasing agent effective for use in increasing BDNF serum level in a human subject.
Owner:ACTIVE BIOTECH AB
Use of laquinimod for reducing fatigue, improving functional status, and improving quality of life in multiple sclerosis patients
InactiveUS20120142730A1Reducing and inhibiting progressionReduce or inhibit progression of the level of fatigueBiocideNervous disorderLaquinimodHuman patient
The subject invention provides methods for reducing or inhibiting progression of the level of fatigue in a multiple sclerosis human patient, for improving or inhibiting deterioration of the functional status of a multiple sclerosis human patient, and for improving or inhibiting deterioration of the general health of a multiple sclerosis human patient, comprising orally administering to the human patient laquinimod or a pharmaceutically acceptable salt thereof. The subject invention also provides a method for providing neuroprotection to a human subject, the method comprising orally administering to the human subject laquinimod or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Treatment of lupus arthritis using laquinimod
This invention provides a method of treating a subject afflicted with active lupus arthritis comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof effective to treat the subject. This invention also provides laquinimod or pharmaceutically acceptable salt thereof for use in treating a subject afflicted with active lupus arthritis. This invention further provides a pharmaceutical composition comprising an amount of laquinimod or pharmaceutically acceptable salt thereof for use in treating a subject afflicted with lupus arthritis.
Owner:TEVA PHARMA IND LTD
Treatment of lupus nephritis using laquinimod
This invention provides a method of treating a subject afflicted with active lupus nephritis comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof effective to treat the subject. This invention also provides laquinimod or pharmaceutically acceptable salt thereof for use in treating a subject afflicted with active lupus nephritis. This invention further provides a pharmaceutical composition comprising an amount of laquinimod or pharmaceutically acceptable salt thereof for use in treating a subject afflicted with active lupus nephritis.
Owner:TEVA PHARMA IND LTD
Treatment of rheumatoid arthritis with a combination of laquinimod and methotrexate
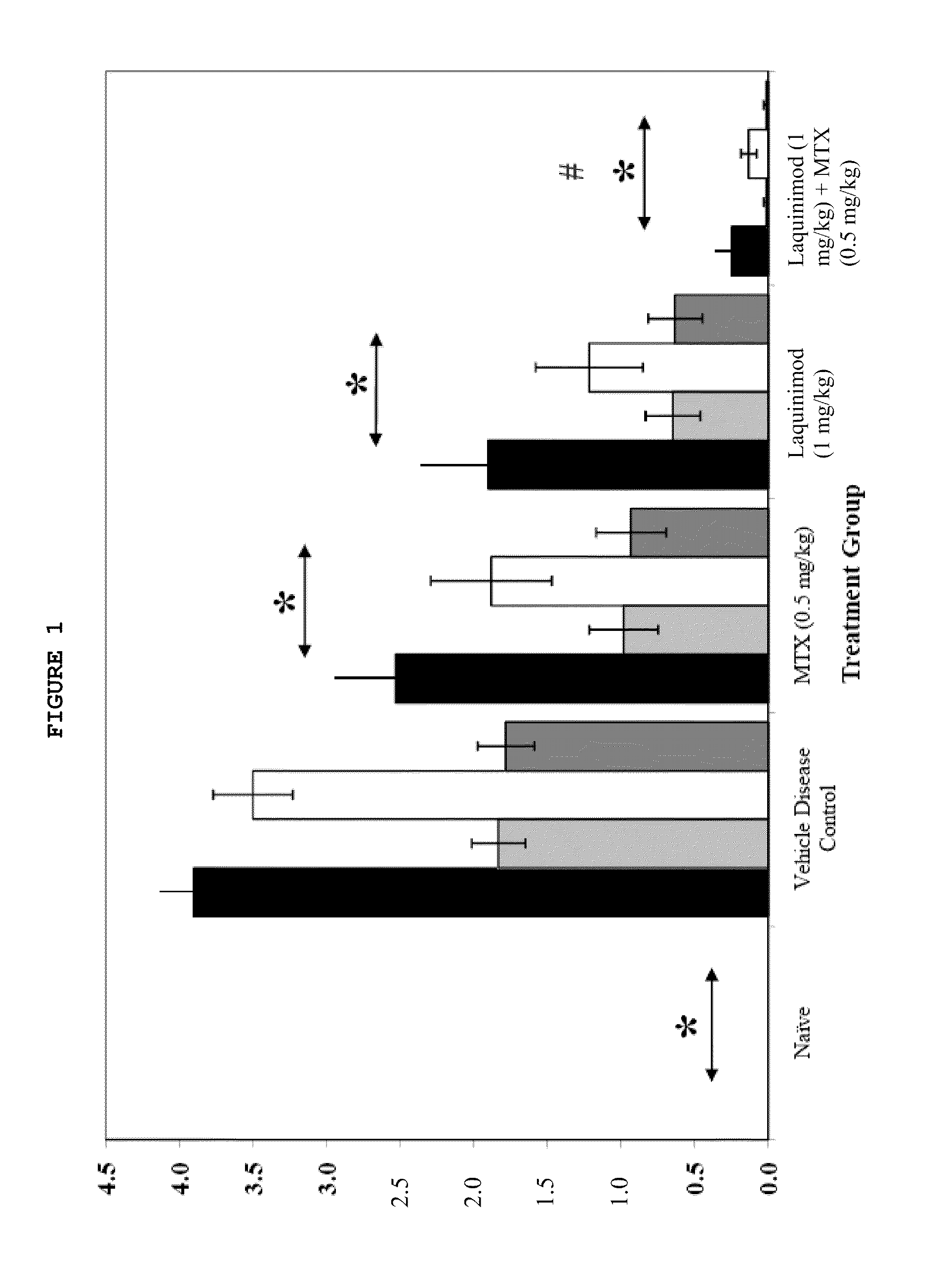
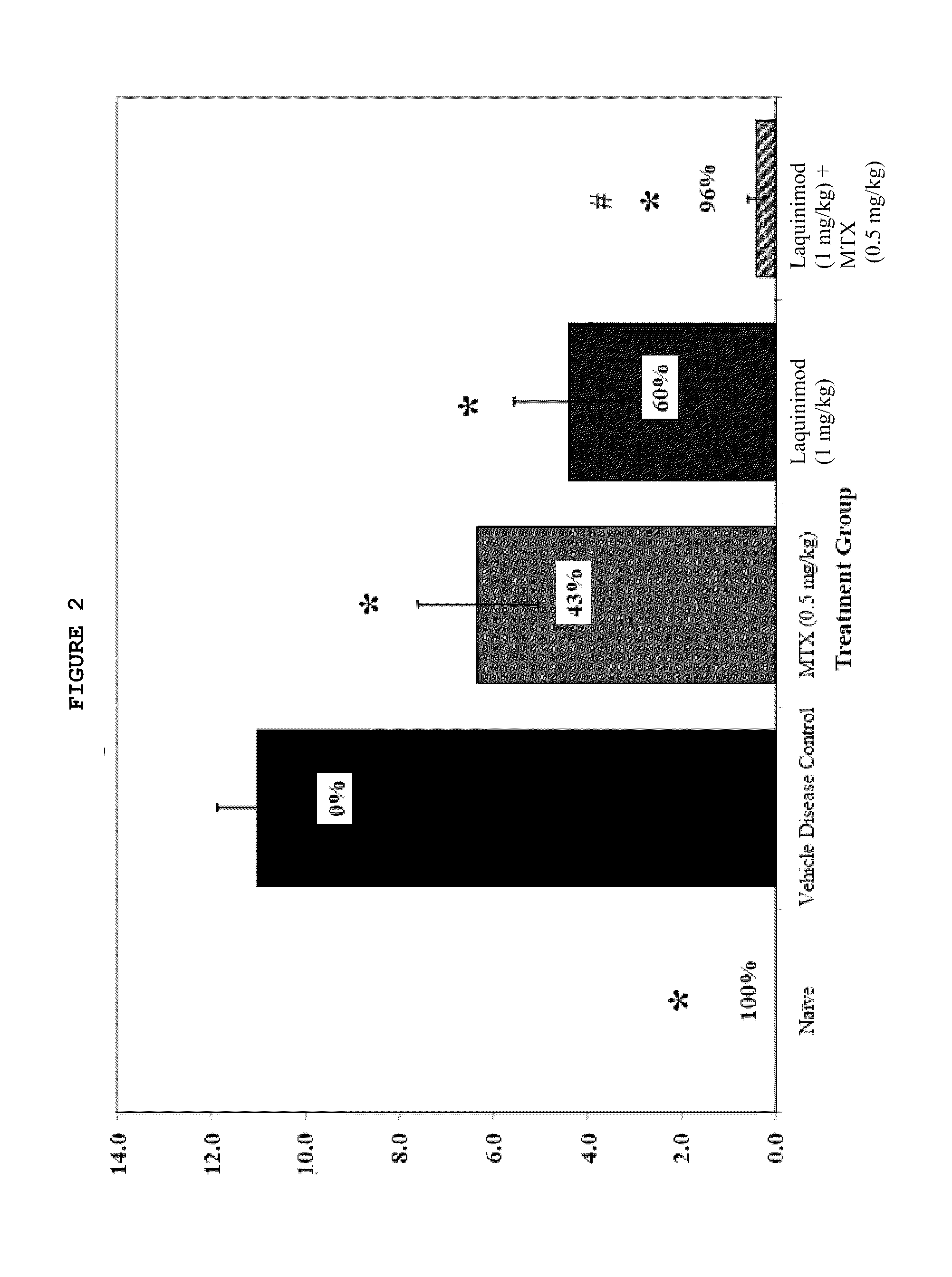
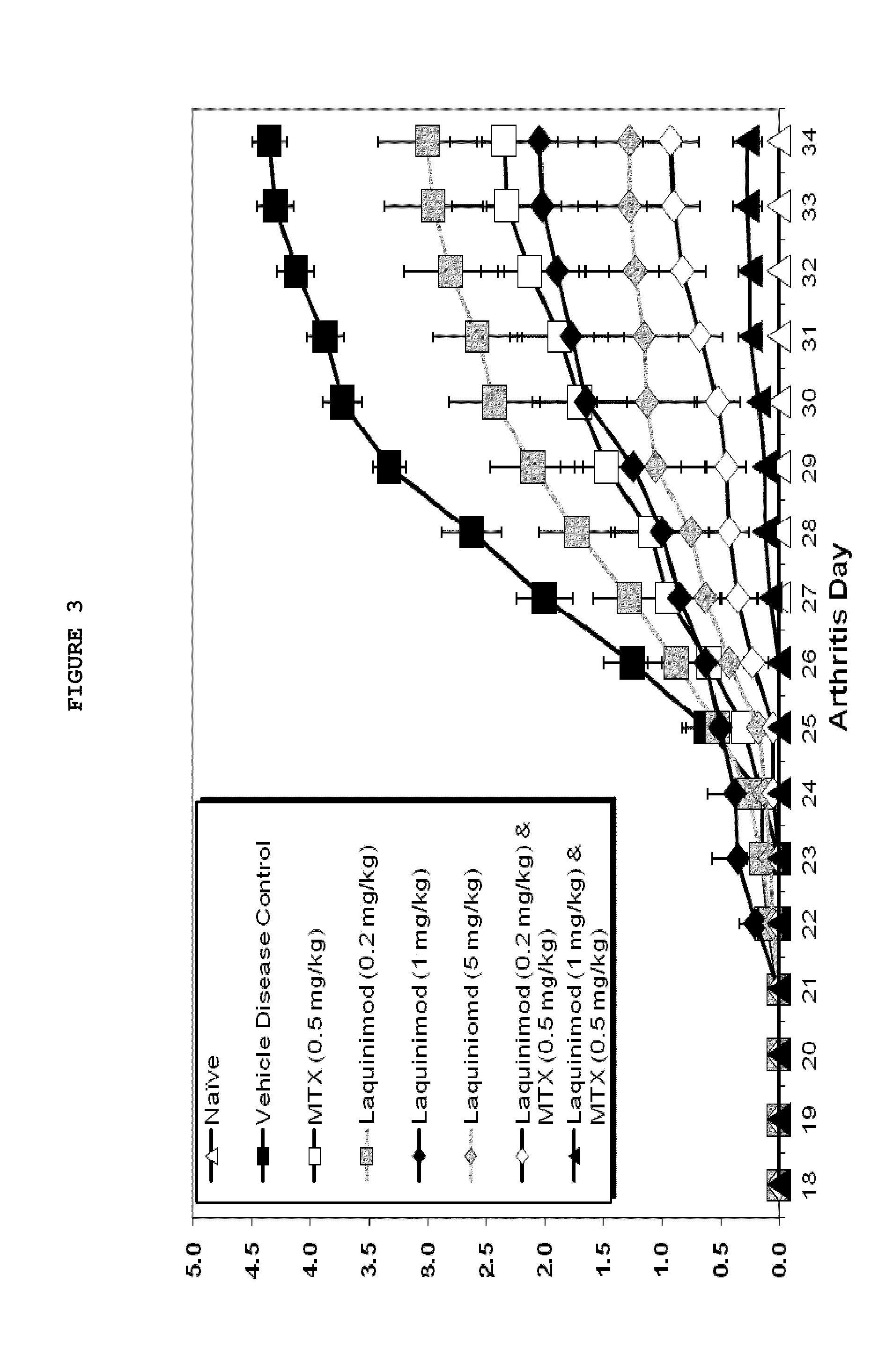
This invention provides a method of treating a subject afflicted with rheumatoid arthritis comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof and an amount of methotrexate, wherein the amounts when taken together are effective to treat the subject. This invention also provides laquinimod or pharmaceutically acceptable salt thereof for use in combination with methotrexate in treating a subject afflicted with rheumatoid arthritis. This invention also provides a pharmaceutical composition comprising an amount of laquinimod or pharmaceutically acceptable salt thereof and an amount of methotrexate for use in treating a subject afflicted with rheumatoid arthritis.
Owner:TEVA PHARMA IND LTD
Combination of laquinimod and pridopidine for treating neurodegenerative disorders, in particular huntington's disease
This invention provides a method of treating a patient afflicted with a neurodegenerative disorder, e.g., Huntington's disease (HD), comprising administering to the patient laquinimod as an add-on therapy to or in combination with pridopidine. This invention also provides a package and a pharmaceutical composition comprising laquinimod and pridopidine for treating a patient afflicted with a neurodegenerative disorder, e.g., HD. This invention also provides laquinimod for use as an add-on therapy or in combination with pridopidine in treating a patient afflicted with a neurodegenerative disorder, e.g., HD. This invention further provides use of laquinimod and pridopidine in the preparation of a combination for treating a patient afflicted with a neurodegenerative disorder, e.g., HD.
Owner:TEVA PHARMA IND LTD
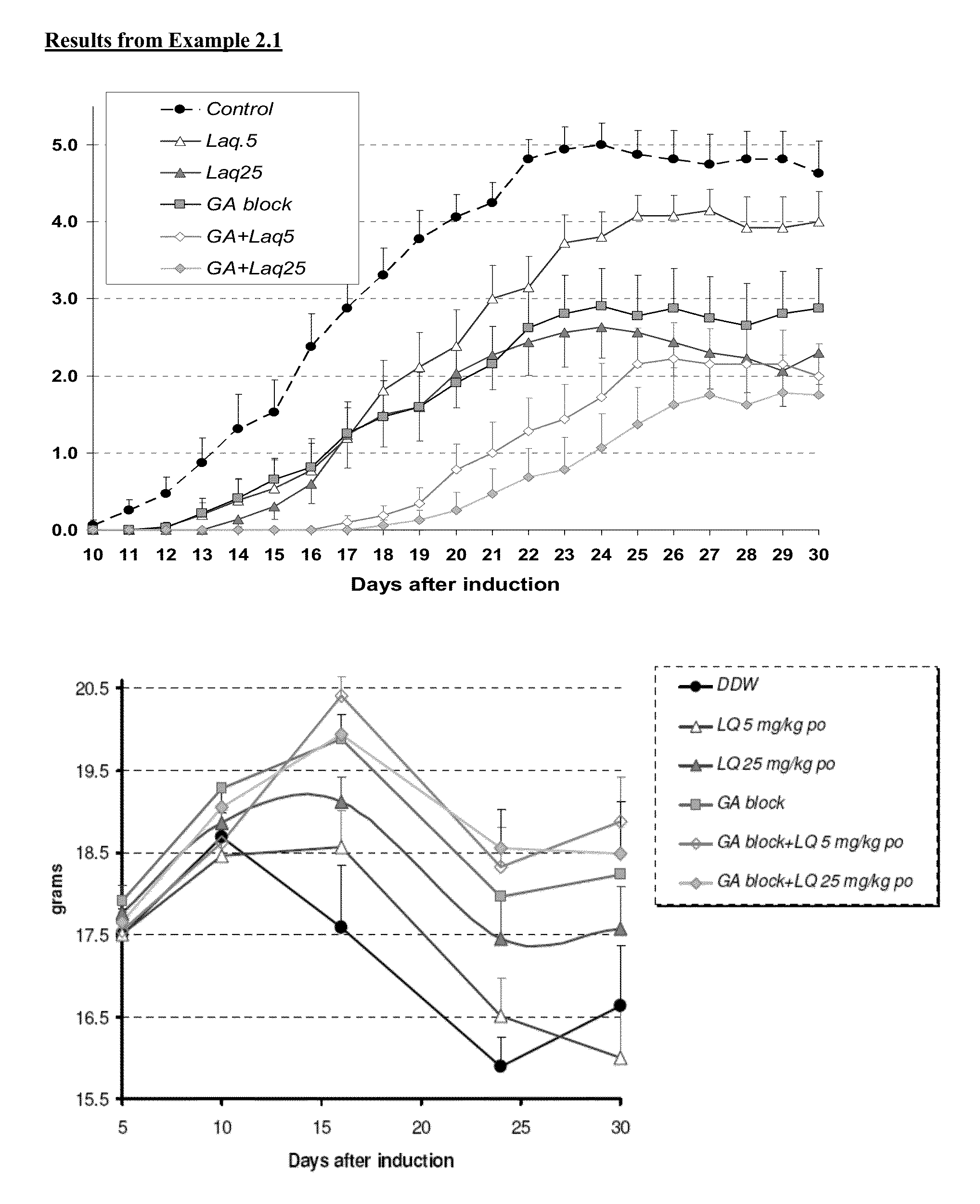
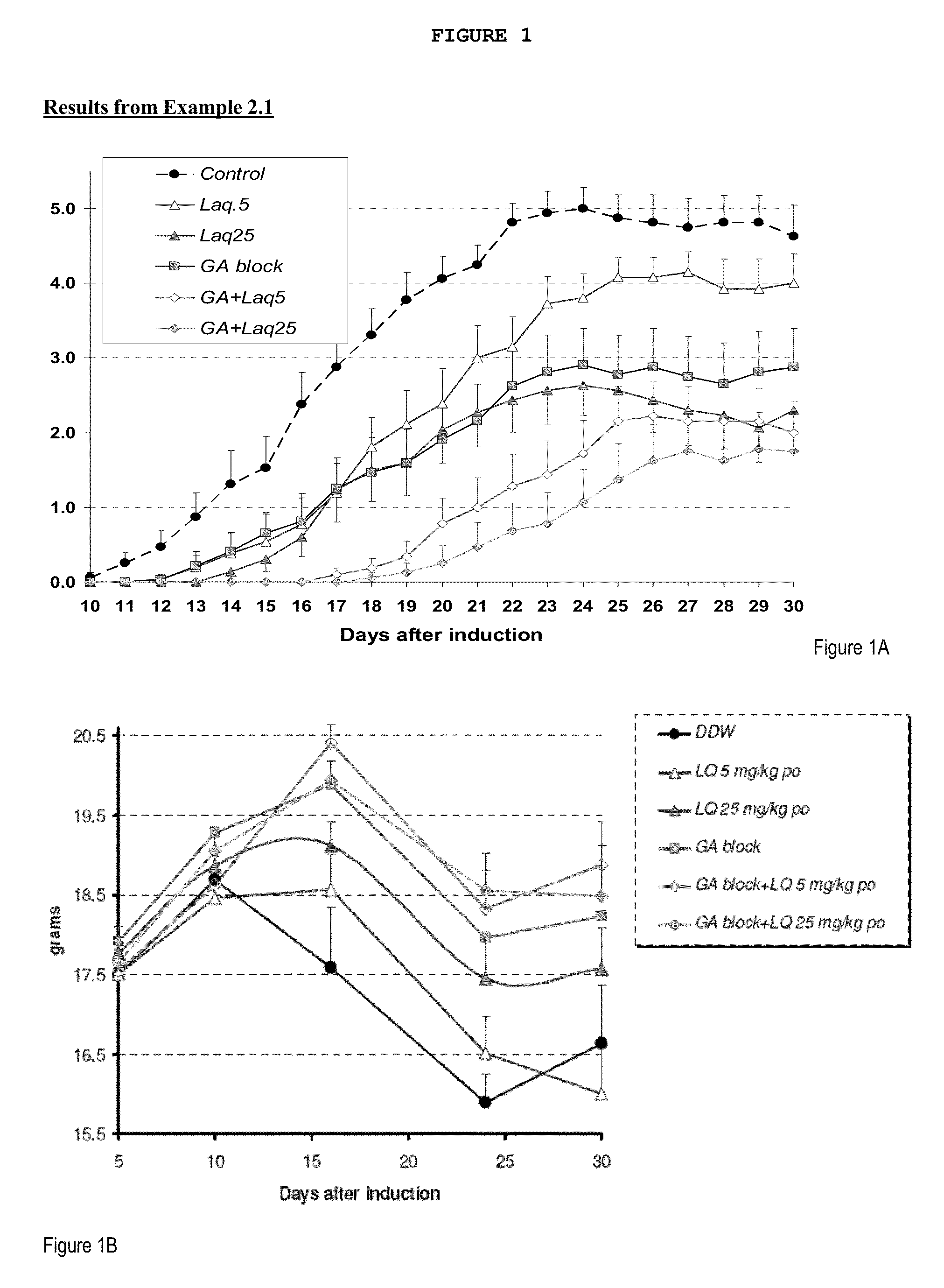
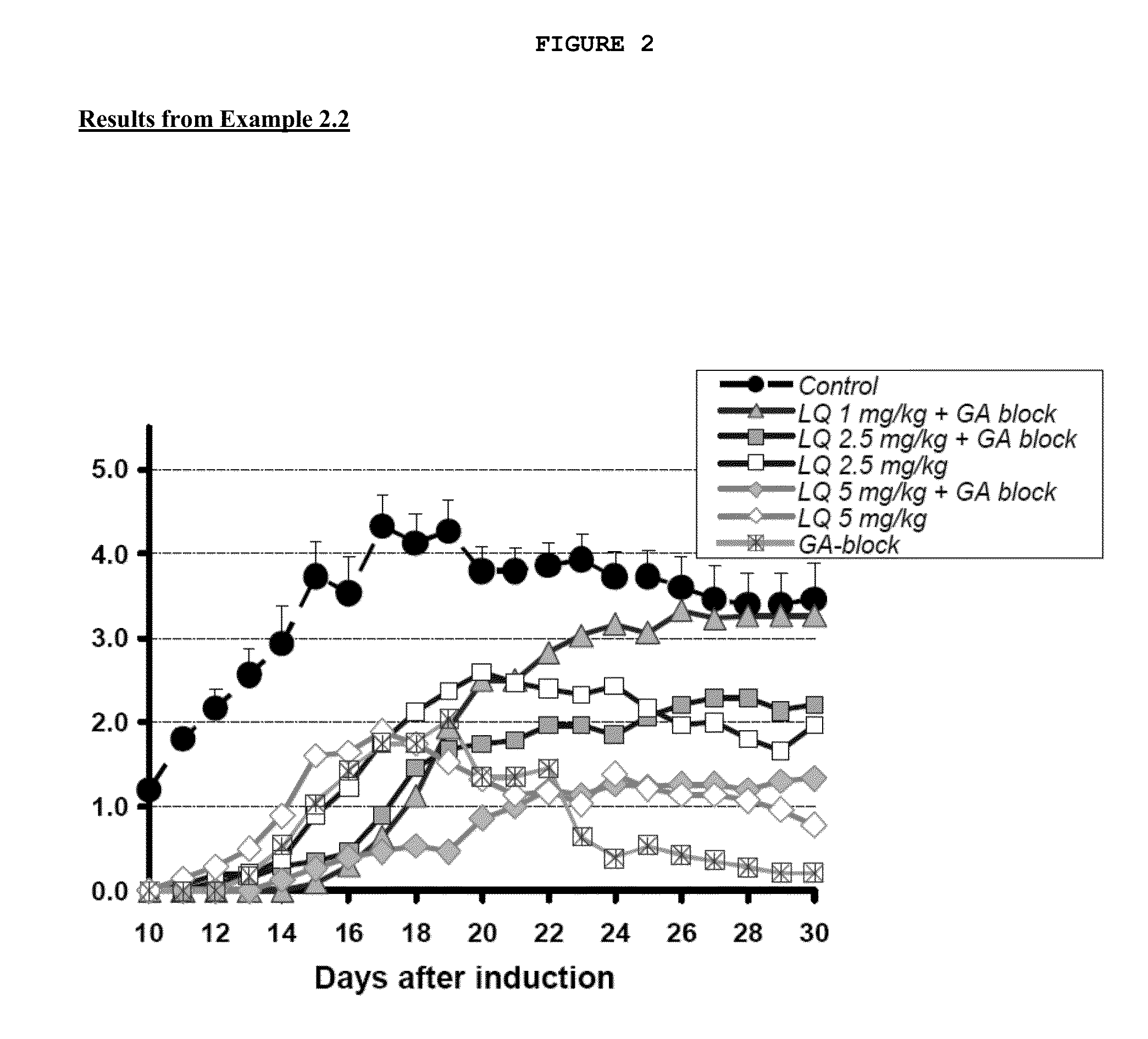
Treatment of multiple sclerosis with combination of laquinimod and glatiramer acetate
InactiveUS20130029916A1Effective treatmentPowder deliverySenses disorderLaquinimodGlatiramer acetate
This invention provides a method of treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the patient laquinimod as an add-on therapy to or in combination with glatiramer acetate. This invention also provides a package and a pharmaceutical composition comprising laquinimod and glatiramer acetate for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with glatiramer acetate in treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and glatiramer acetate in the preparation of a combination for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
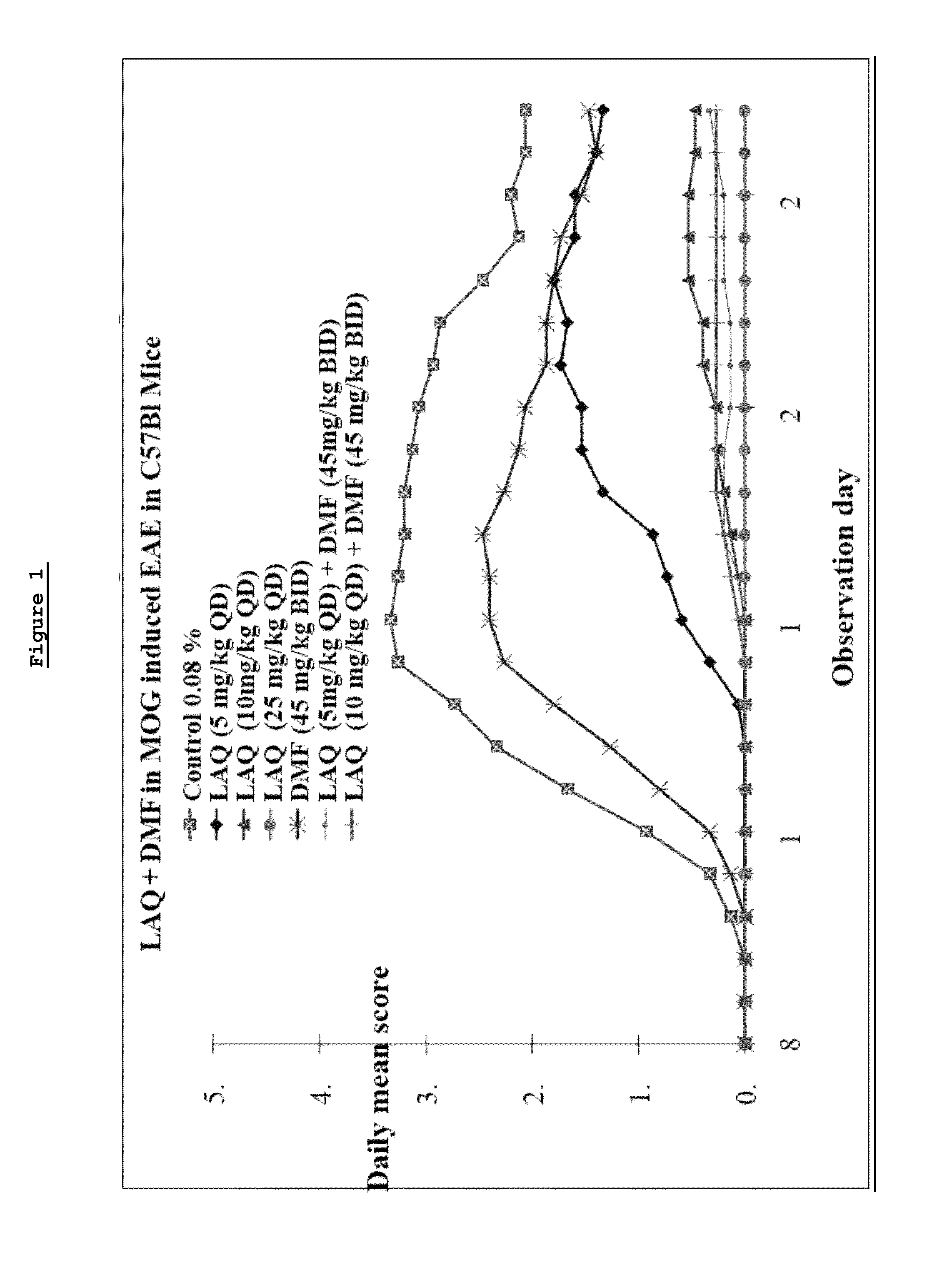
Treatment of multiple sclerosis with combination of laquinimod and dimethyl fumarate
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with DMF. This invention also provides a package comprising laquinimod and DMF for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with DMF in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides a pharmaceutical composition comprising laquinimod and DMF for use in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and DMF in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
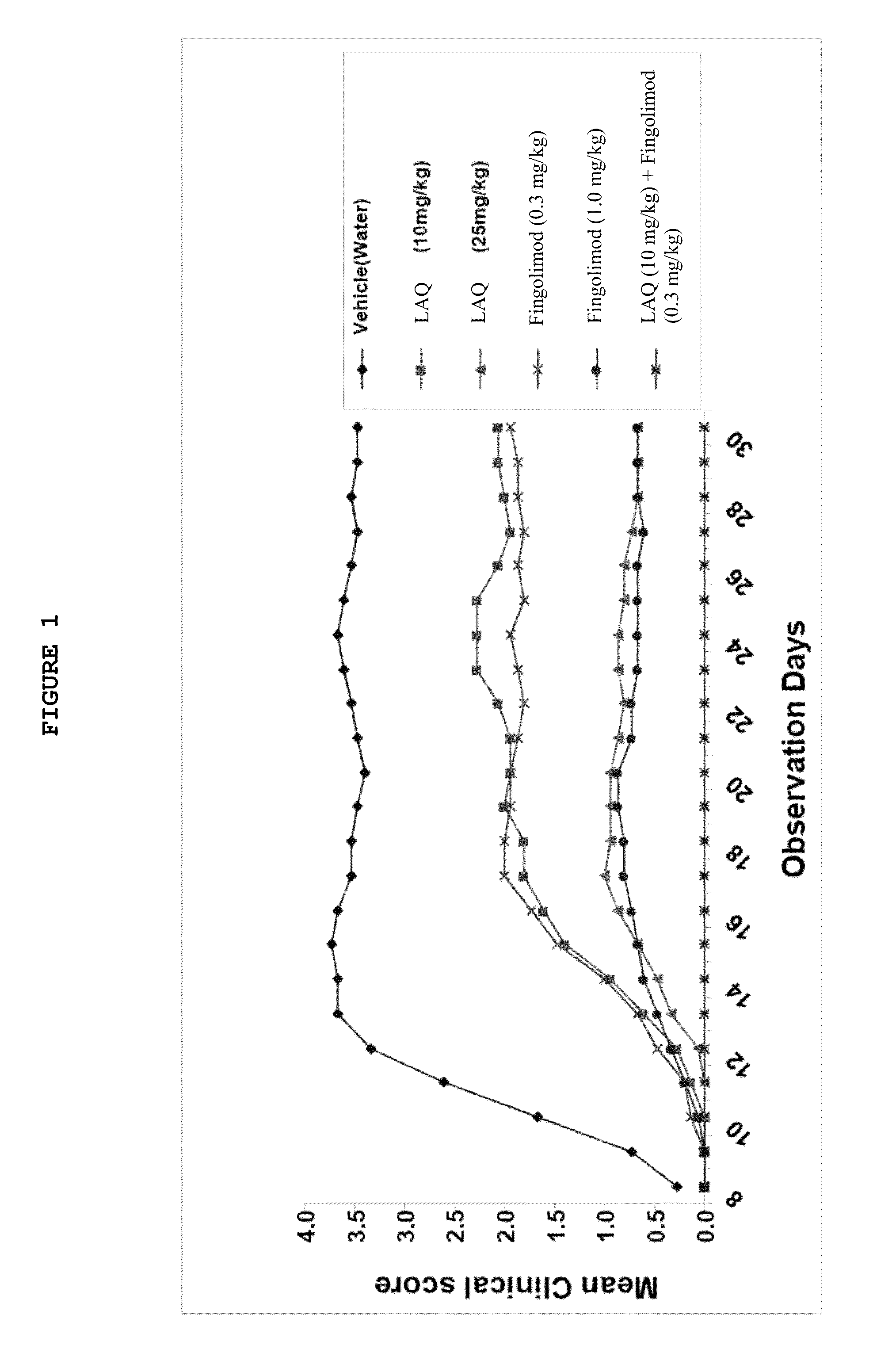
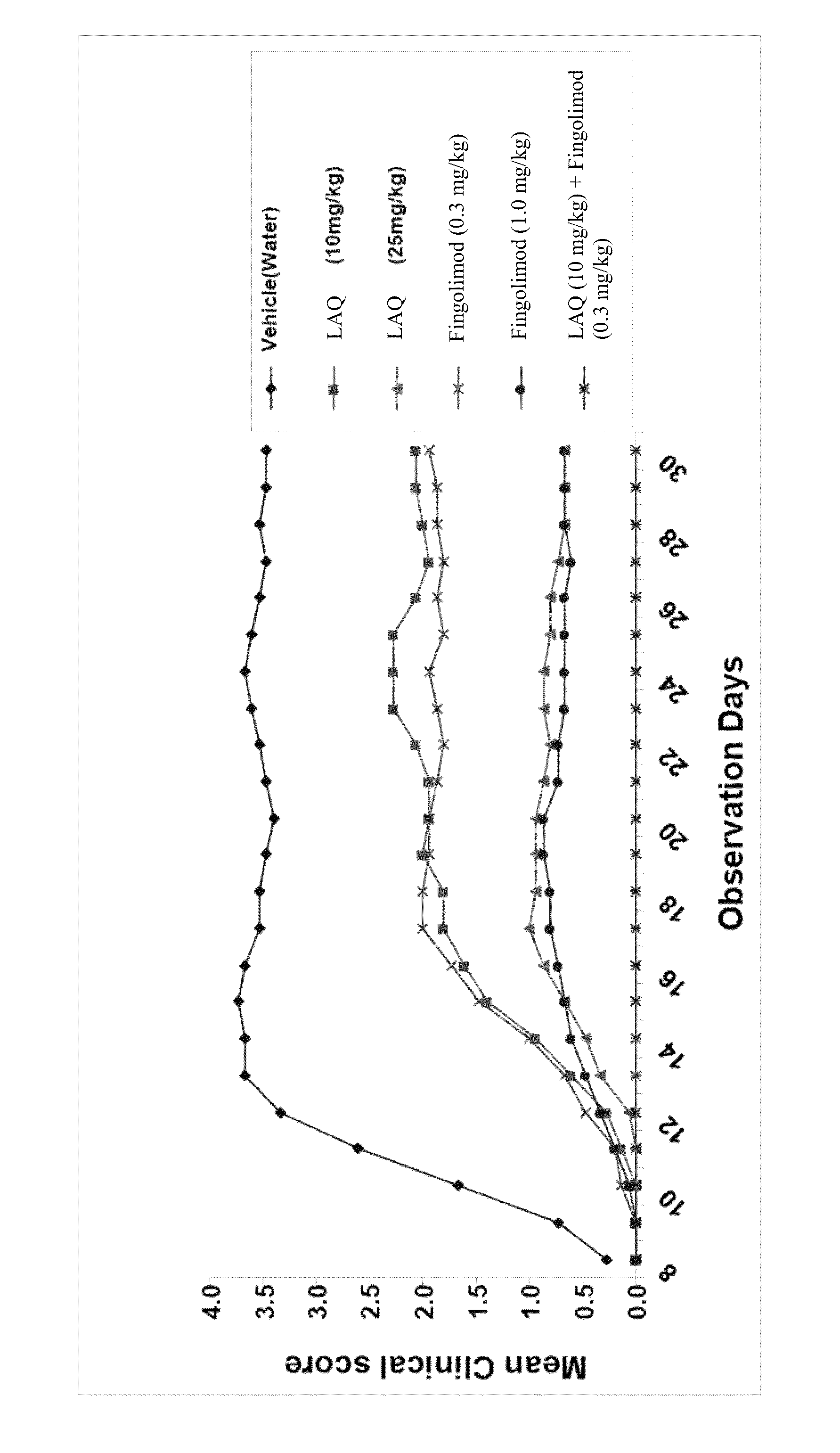
Treatment Of Multiple Sclerosis With Combination Of Laquinimod And Fingolimod
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with fingolimod. This invention also provides a package and a pharmaceutical composition comprising laquinimod and fingolimod for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with fingolimod in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and fingolimod in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Use of laquinimod for treating crohn's disease patients who failed first-line Anti-tnf therapy
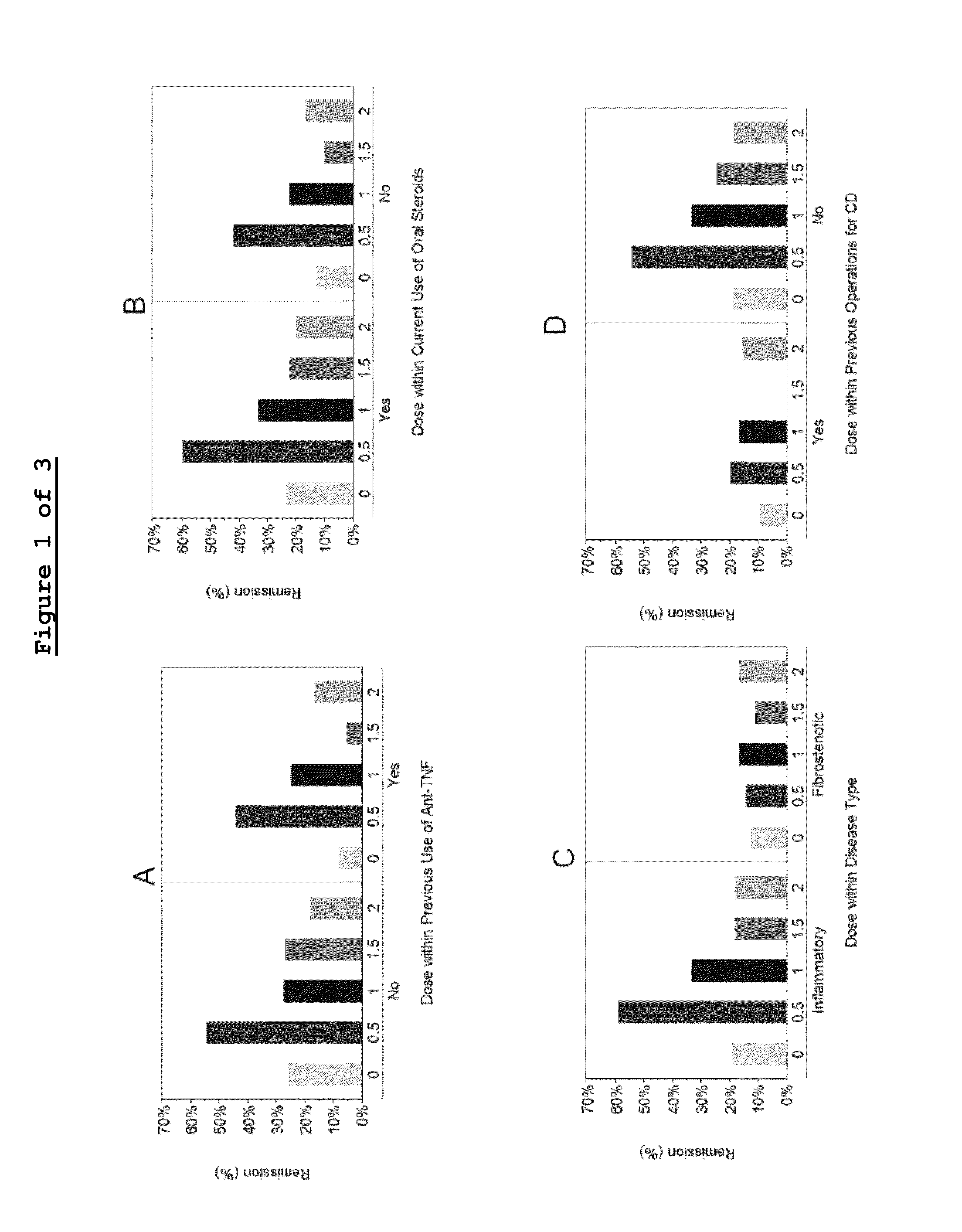
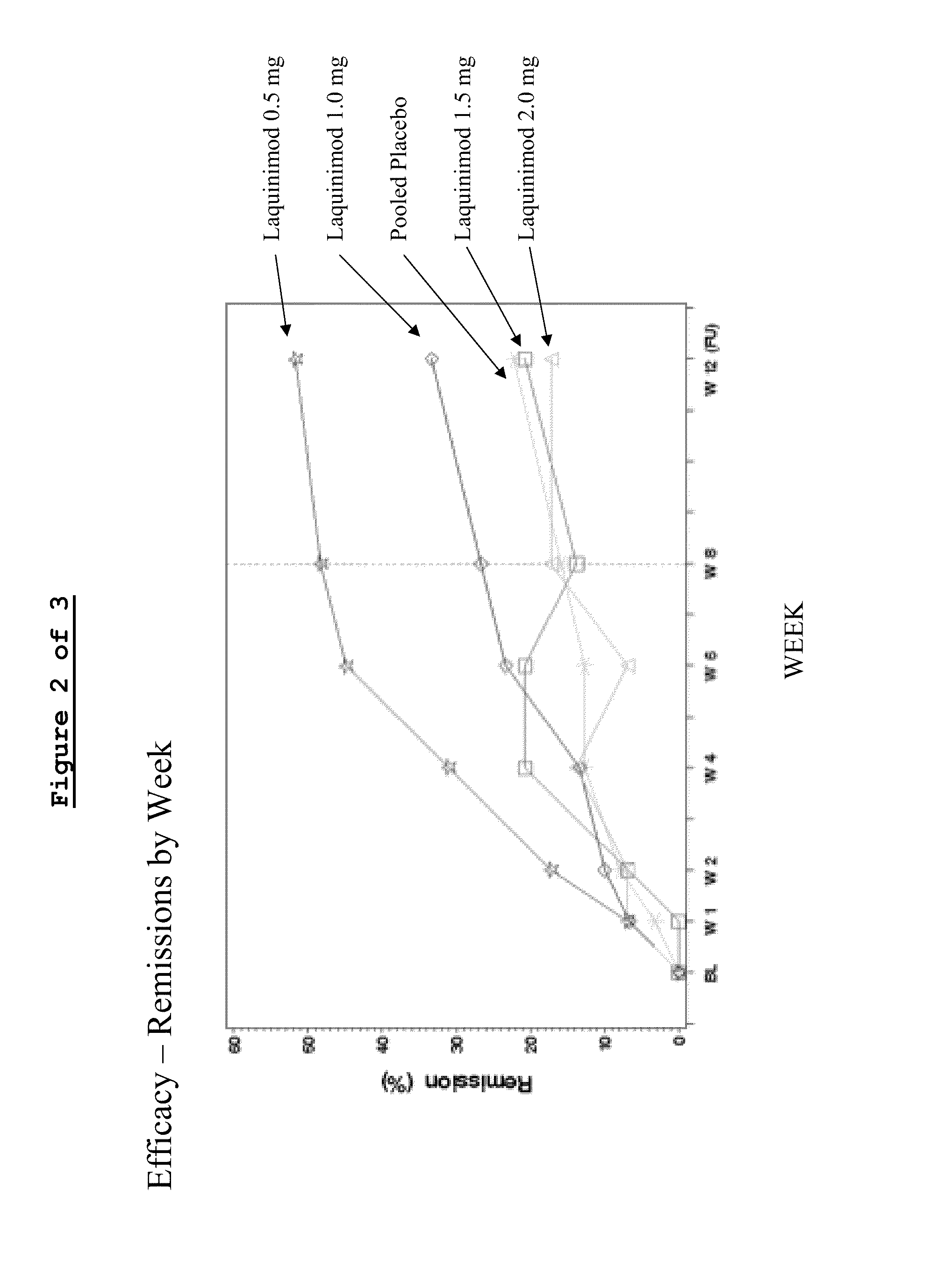
This application provides for a method of treating a human patient afflicted with anti-TNFα refractory Crohn's disease, of treating a human patient afflicted with non-fibrostenotic Crohn's disease, and of treating a human patient whose Crohn's disease had not been surgically treated, the method comprising periodically administering to the patient an amount of laquinimod or pharmaceutically acceptable salt thereof effective to treat the patient. This application also provides for a method of inducing or maintaining clinical remission in a human patient afflicted with Crohn's disease comprising periodically administering to the patient an amount of laquinimod effective to induce or maintain clinical remission in the patient, which amount of laquinimod is less than 0.5 mg / day.
Owner:ACTIVE BIOTECH AB
Combination of laquinimod and pridopidine for treating neurodegenerative disorders, in particular huntington's disease
This invention provides a method of treating a patient afflicted with a neurodegenerative disorder, e.g., Huntington's disease (HD), comprising administering to the patient laquinimod as an add-on therapy to or in combination with pridopidine. This invention also provides a package and a pharmaceutical composition comprising laquinimod and pridopidine for treating a patient afflicted with a neurodegenerative disorder, e.g., HD. This invention also provides laquinimod for use as an add-on therapy or in combination with pridopidine in treating a patient afflicted with a neurodegenerative disorder, e.g., HD. This invention further provides use of laquinimod and pridopidine in the preparation of a combination for treating a patient afflicted with a neurodegenerative disorder, e.g., HD.
Owner:TEVA PHARM USA INC
Use of high dose laquinimod for treating multiple sclerosis
InactiveUS20130303569A1Extension of timeReducing brain atrophyBiocideNervous disorderHigh dosesLaquinimod
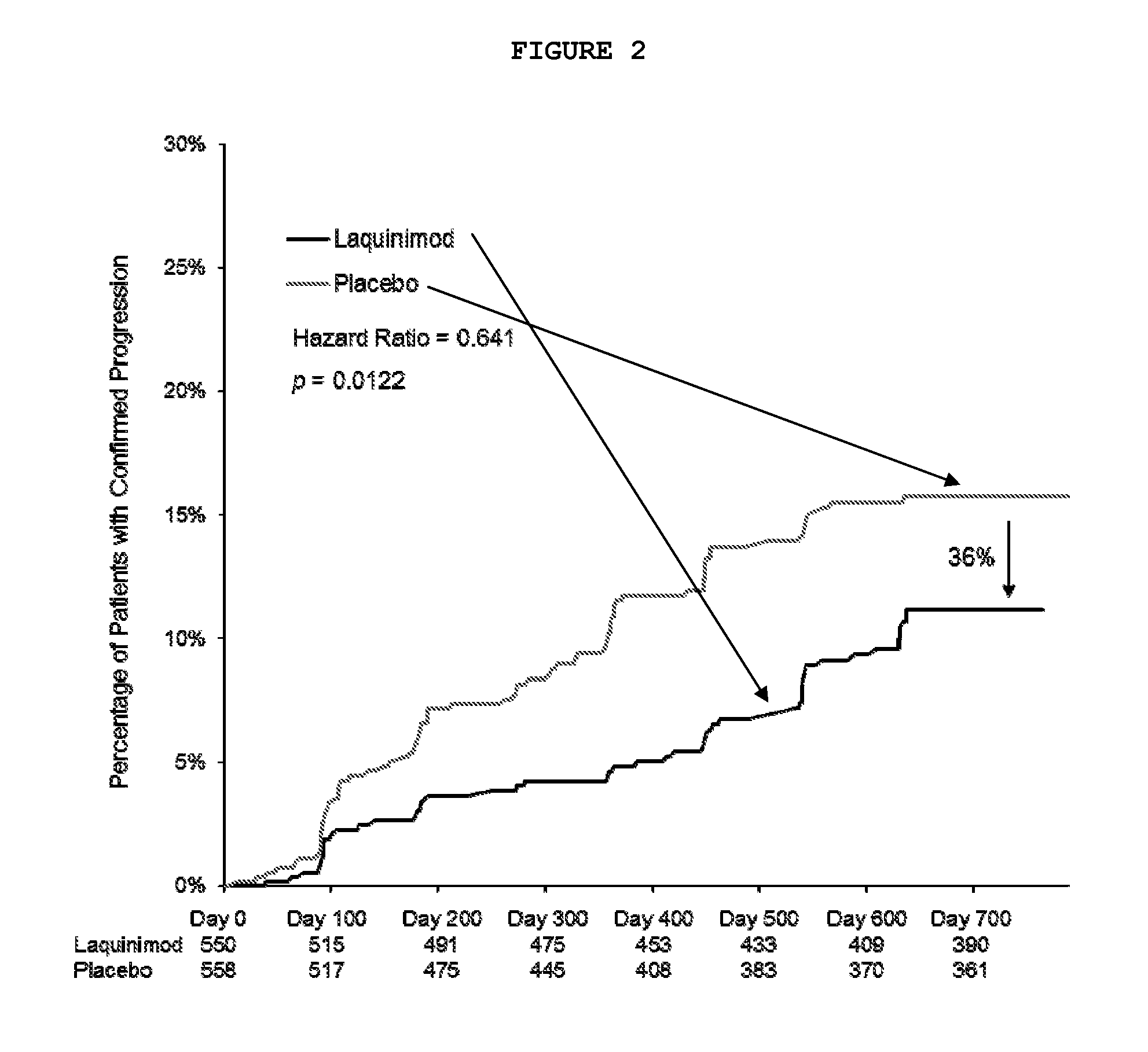
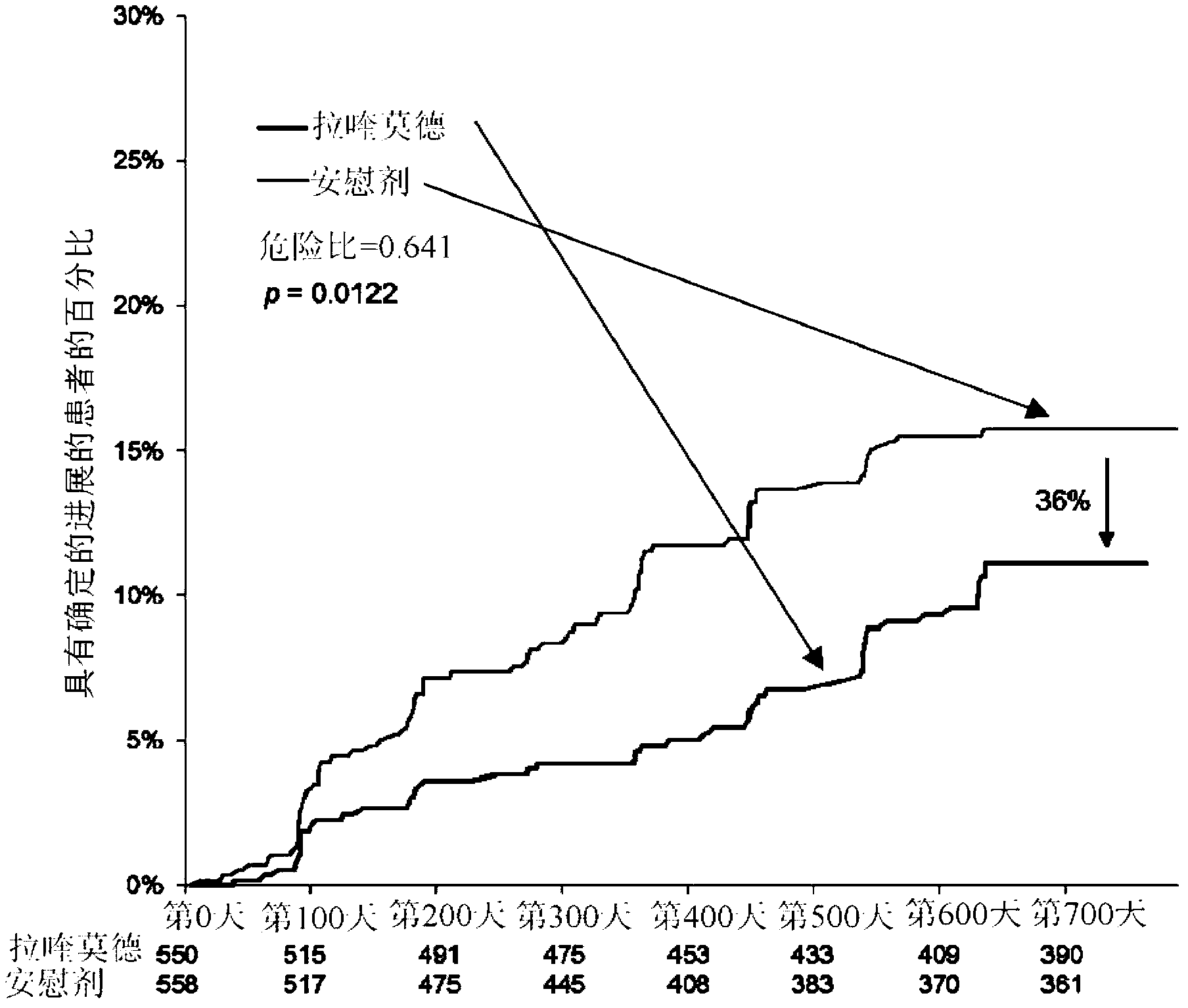
Disclosed herein are methods of treating a human patient afflicted with multiple sclerosis (MS) or presenting a clinically isolated syndrome (CIS), for treating a human subject by providing neuroprotection, and of treating a human patient afflicted with MS or presenting a CIS by increasing the time to confirmed disease progression and / or confirmed relapse or reducing brain atrophy, comprising orally administering a daily dose of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof. Also disclosed is a pharmaceutical oral unit dosage form of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier for use in treating a human patient afflicted with MS or presenting a CIS, in treating a human subject by providing neuroprotection, or in treating a human patient afflicted with MS or presenting a CIS by increasing the time to confirmed disease progression and / or confirmed relapse or reducing brain atrophy.
Owner:TEVA PHARMA IND LTD
Laquinimod for reducing thalamic damage in multiple sclerosis
InactiveUS20140107154A1Inhibiting and reducing thalamic damageReduce harmBiocideNervous disorderDiseaseThalamus
This invention provides methods for inhibiting or reducing thalamic damage in a subject comprising administering to the subject an amount of laquinimod, wherein the subject is a human patient afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome who has been determined to have thalamic damage at baseline, a subject afflicted with a disease or disorder other than a form of multiple sclerosis or a clinically isolated syndrome, or a subject not afflicted with a form of multiple sclerosis or a presenting clinically isolated syndrome, and laquinimod and laquinimod pharmaceutical compositions for use thereof. This invention also provides methods for inhibiting or reducing tremor or spasticity in a subject afflicted by tremor or spasticity, comprising administering to the subject an amount of laquinimod, and laquinimod and laquinimod pharmaceutical compositions for use thereof.
Owner:TEVA PHARMA IND LTD
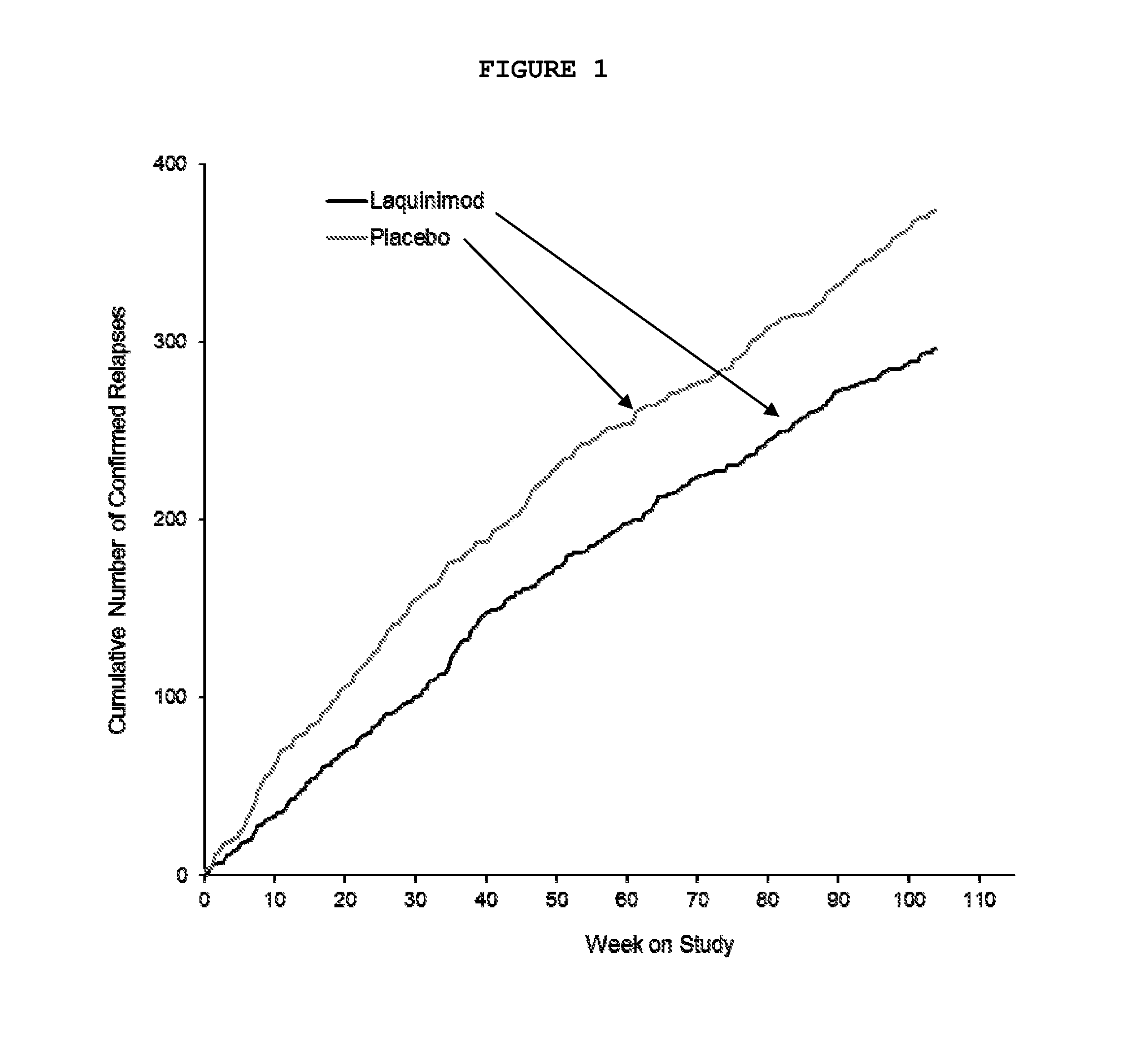
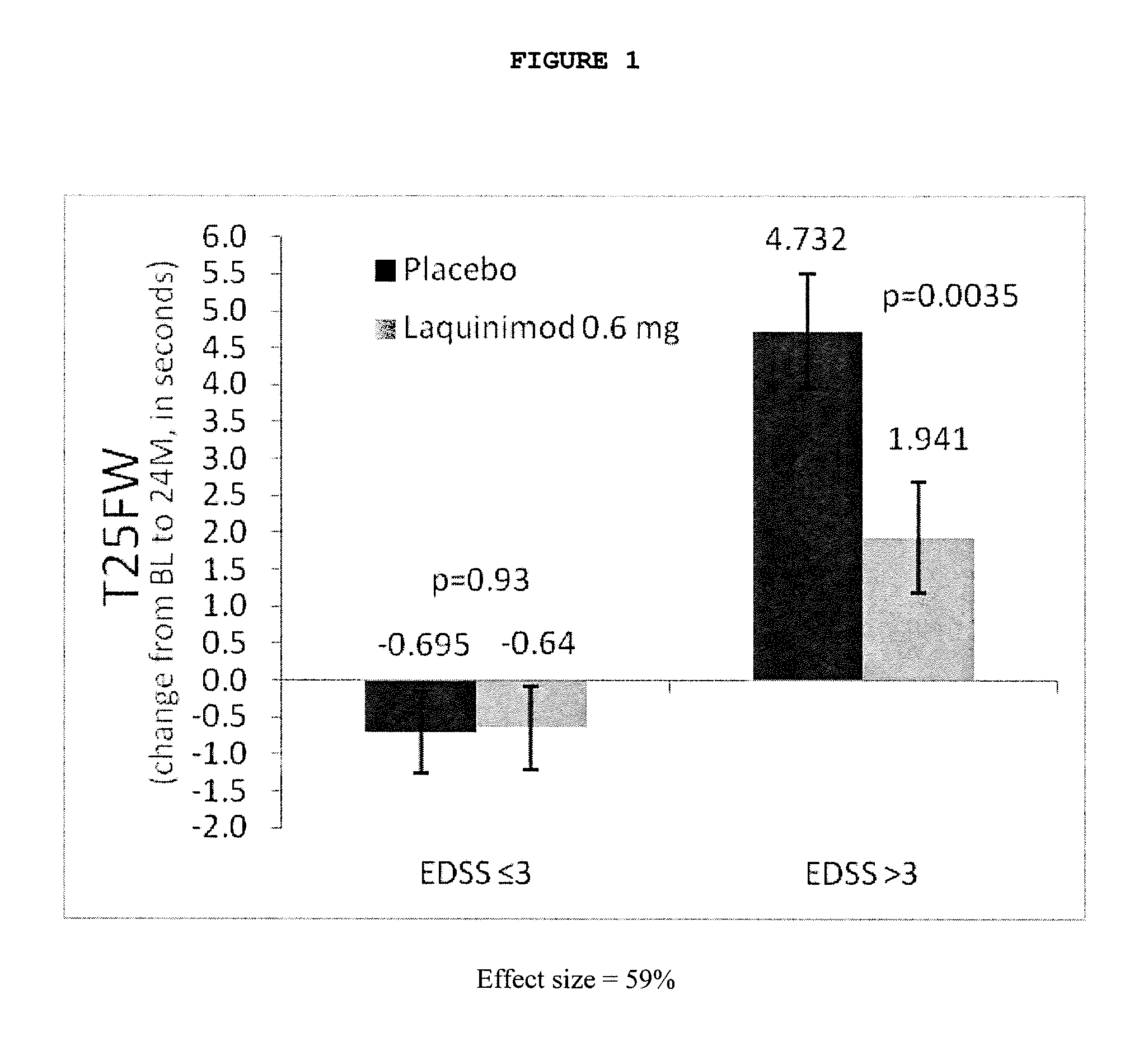
Treatment of multiple sclerosis with laquinimod
The subject invention provides for methods of reducing the relapse rate and / or reducing the accumulation of physical disability in a relapsing-remitting multiple sclerosis human patient, the method comprising orally administering to the patient a daily dose of 0.6 mg laquinimod.The subject invention also provides for pharmaceutical oral unit dosage forms of 0.6 mg laquinimod for use in reducing the relapse rate and / or for use in reducing the accumulation of physical disability in a relapsing-remitting multiple sclerosis human patient.
Owner:TEVA PHARMA IND LTD
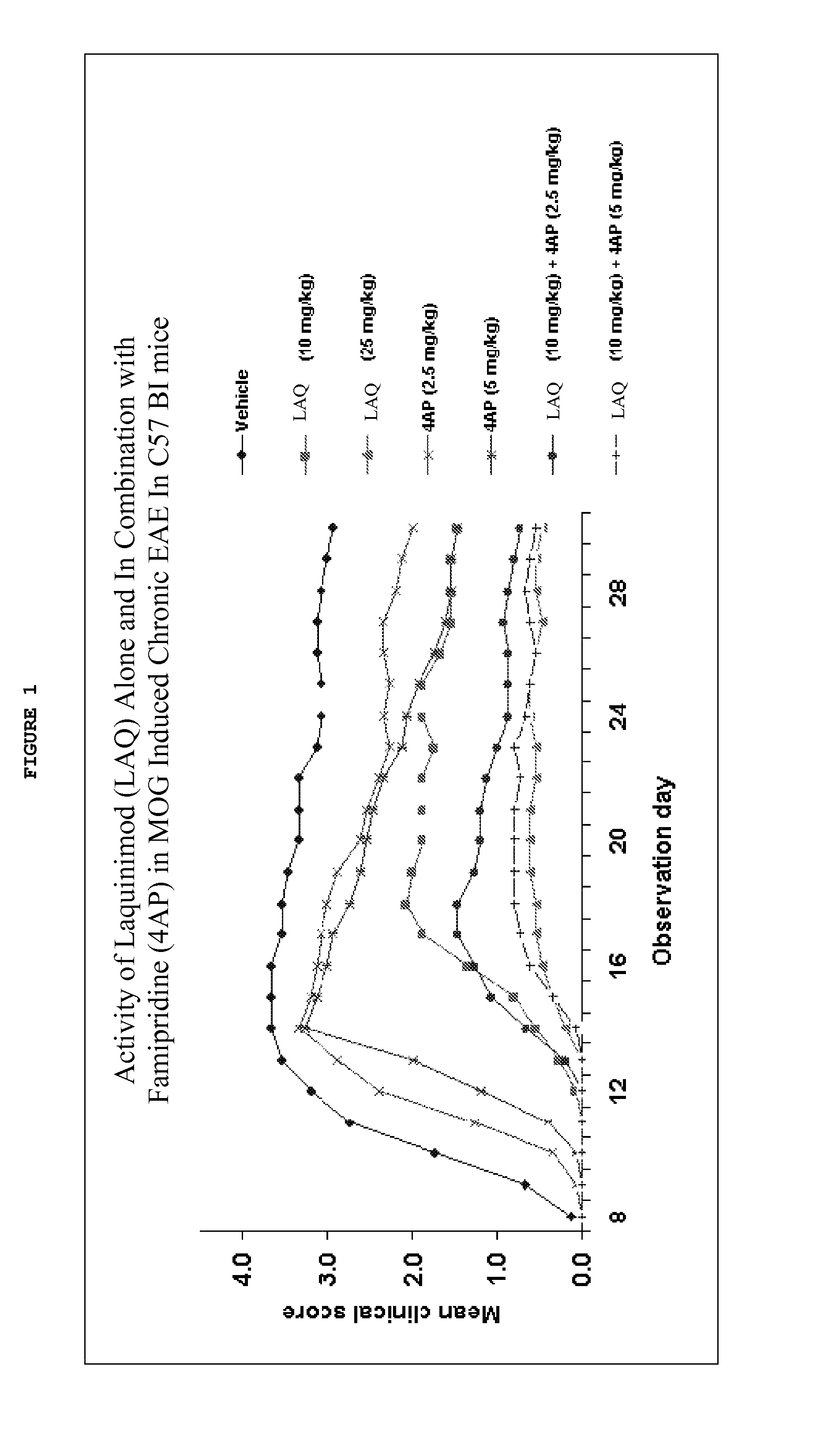
Treatment of multiple sclerosis with combination of laquinimod and fampridine
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject fampridine as an add-on therapy to or in combination with laquinimod. This invention also provides a package comprising laquinimod and fampridine for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides fampridine for use as an add-on therapy or in combination with laquinimod in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides a pharmaceutical composition comprising laquinimod and fampridine for use in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and fampridine in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Combinations comprising siponimod and laquinimod for the treatment of multiple sclerosis
ActiveUS20170304289A1Benefit in treatmentPrevent benefitOrganic active ingredientsNervous disorderSiponimodLaquinimod
The present invention relates to combinations and pharmaceutical compositions comprising siponimod and laquinimod, and the use of said combinations and / or compositions for the treatment of multiple sclerosis, particularly secondary progressive multiple sclerosis.
Owner:NOVARTIS AG
Gene expression biomarkers of laquinimod responsiveness
This invention provides a method of predicting clinical responsiveness to laquinimod therapy in a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising evaluating expression of a biomarker in the subject. This invention also provides a method of treating said subject comprising determining whether the subject is a laquinimod-responder by evaluating expression of a biomarker. Also provided is laquinimod or a pharmaceutical composition comprising laquinimod for use in treating said subject, and a therapeutic package for use in dispensing to said subject, wherein the subject has been identified as a laquinimod-responder or expression of a biomarker in the subject is up-regulated or suppressed.
Owner:TEL HASHOMER MEDICAL RES INFRASTRUCTURE & SERVICES +1
Use of laquinimod for reducing fatigue, improving functional status, and improving quality of life in multiple sclerosis patients
The subject invention provides methods for reducing or inhibiting progression of the level of fatigue in a multiple sclerosis human patient, for improving or inhibiting deterioration of the functional status of a multiple sclerosis human patient, and for improving or inhibiting deterioration of the general health of a multiple sclerosis human patient, comprising orally administering to the human patient laquinimod or a pharmaceutically acceptable salt thereof. The subject invention also provides a method for providing neuroprotection to a human subject, the method comprising orally administering to the human subject laquinimod or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Treatment of ocular inflammatory diseases using laquinimod
InactiveUS20130324574A1Improve oral bioavailabilityBiocideSenses disorderDiseaseViral Conjunctivitis
Disclosed is a method for treating an ocular inflammatory disease (OID), e.g., uveitis or conjunctivitis, comprising periodic administration of a therapeutically effective amount of laquinimod or a pharmaceutically acceptable salt thereof. Also provided is a pharmaceutical composition comprising laquinimod or a pharmaceutically acceptable salt thereof for use in treating a subject suffering from an OID, uveitis, bacterial conjunctivitis, viral conjunctivitis, an inflammation of the orbital tissue, the lacrimal apparatus, the eyelid, the cornea, the retina or the optic pathway. This application also provides a method for treating a subject suffering from an autoimmune disease-associated ocular inflammation comprising periodic ocular administration to the subject a therapeutically effective amount of laquinimod or a pharmaceutically acceptable salt, and an ocular pharmaceutical composition comprising laquinimod or a pharmaceutically acceptable salt thereof for use in treating an autoimmune disease-associated ocular inflammation.
Owner:TEVA PHARMA IND LTD
Treatment of lupus arthritis using laquinimod
This invention provides a method of treating a subject afflicted with active lupus arthritis comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof effective to treat the subject. This invention also provides laquinimod or pharmaceutically acceptable salt thereof for use in treating a subject afflicted with active lupus arthritis. This invention further provides a pharmaceutical composition comprising an amount of laquinimod or pharmaceutically acceptable salt thereof for use in treating a subject afflicted with lupus arthritis.
Owner:TEVA PHARMA IND LTD
Treatment of multiple sclerosis with combination of laquinimod and fingolimod
Owner:TEVA PHARMA IND LTD
Laquinimod for treatment of gaba mediated disorders
This invention provides a method of treating a subject suffering from a GABA related disorder comprising periodically administering to the subject an effective amount of laquinimod or pharmaceutically acceptable salt thereof in an amount effective to treat the subject.
Owner:ACTIVE BIOTECH AB
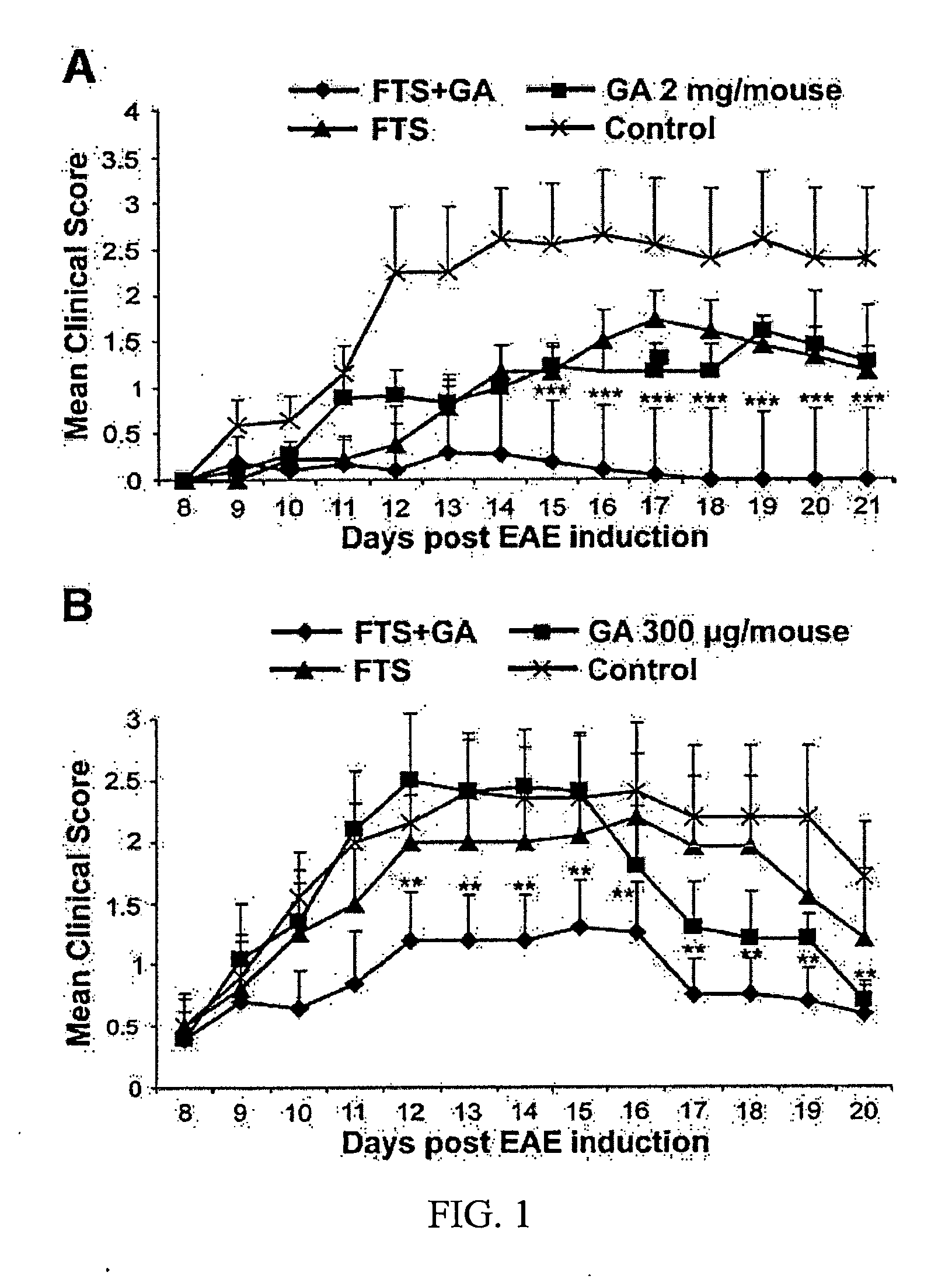
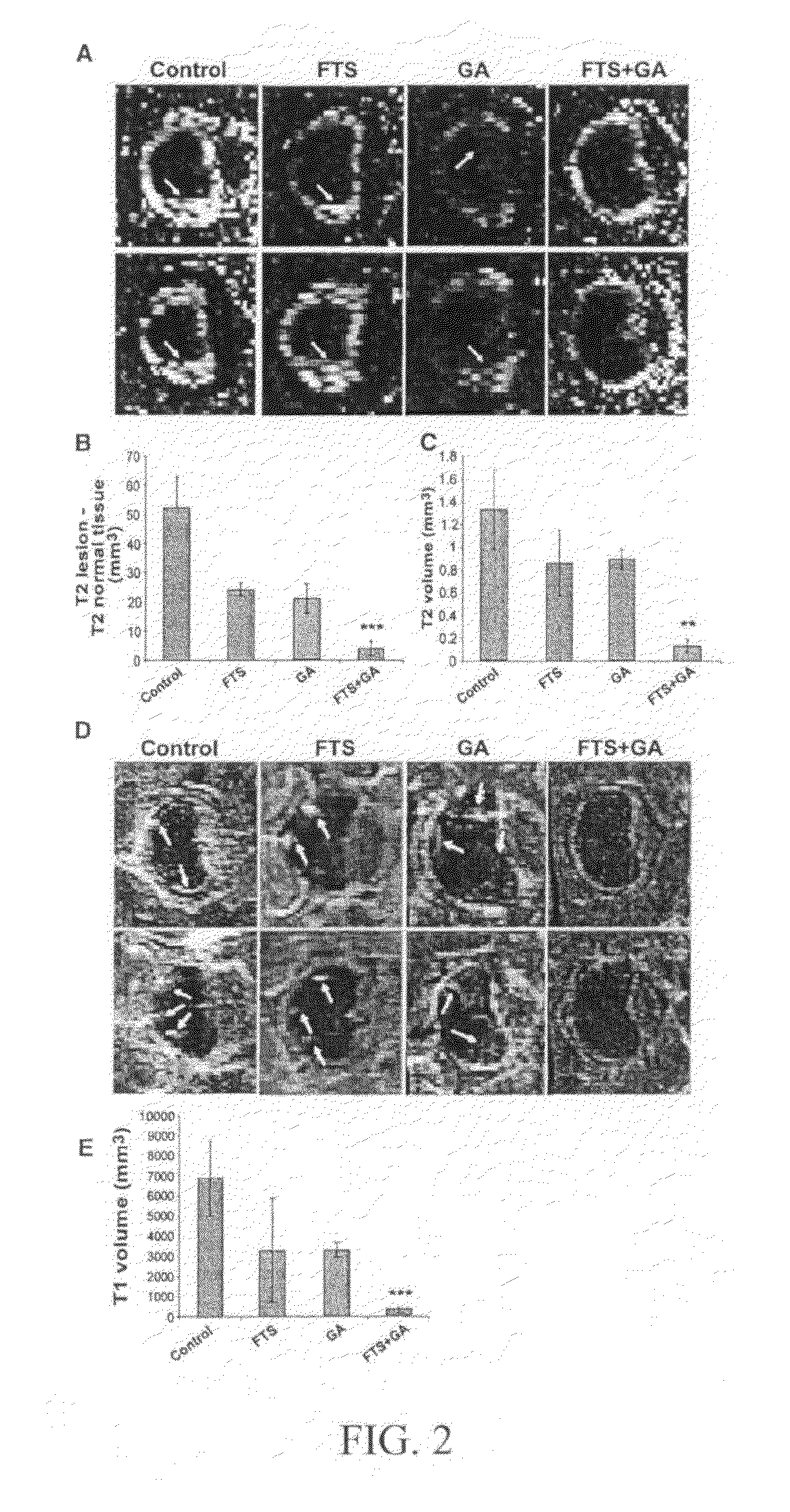
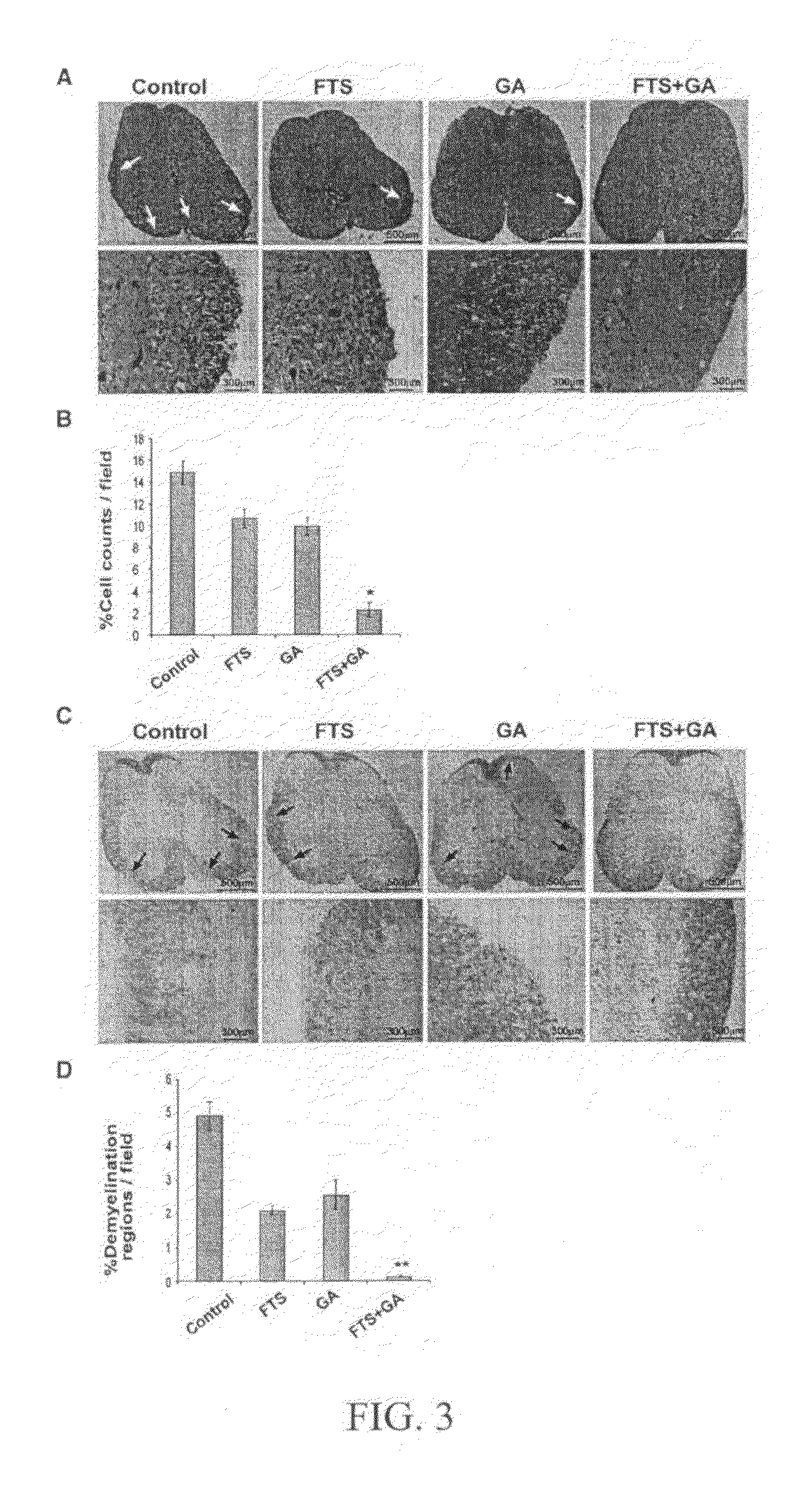
Treatment of multiple sclerosis
Disclosed are methods for treating multiple sclerosis patients that entail co-administration of effective amounts of a Ras antagonist which is farnesylthiosalicylic acid or an analog thereof, and a second active agent selected from glatiramer acetate, laquinimod and combinations thereof. Therapeutic compositions and methods of making them are also disclosed.
Owner:RAMOT AT TEL AVIV UNIV LTD
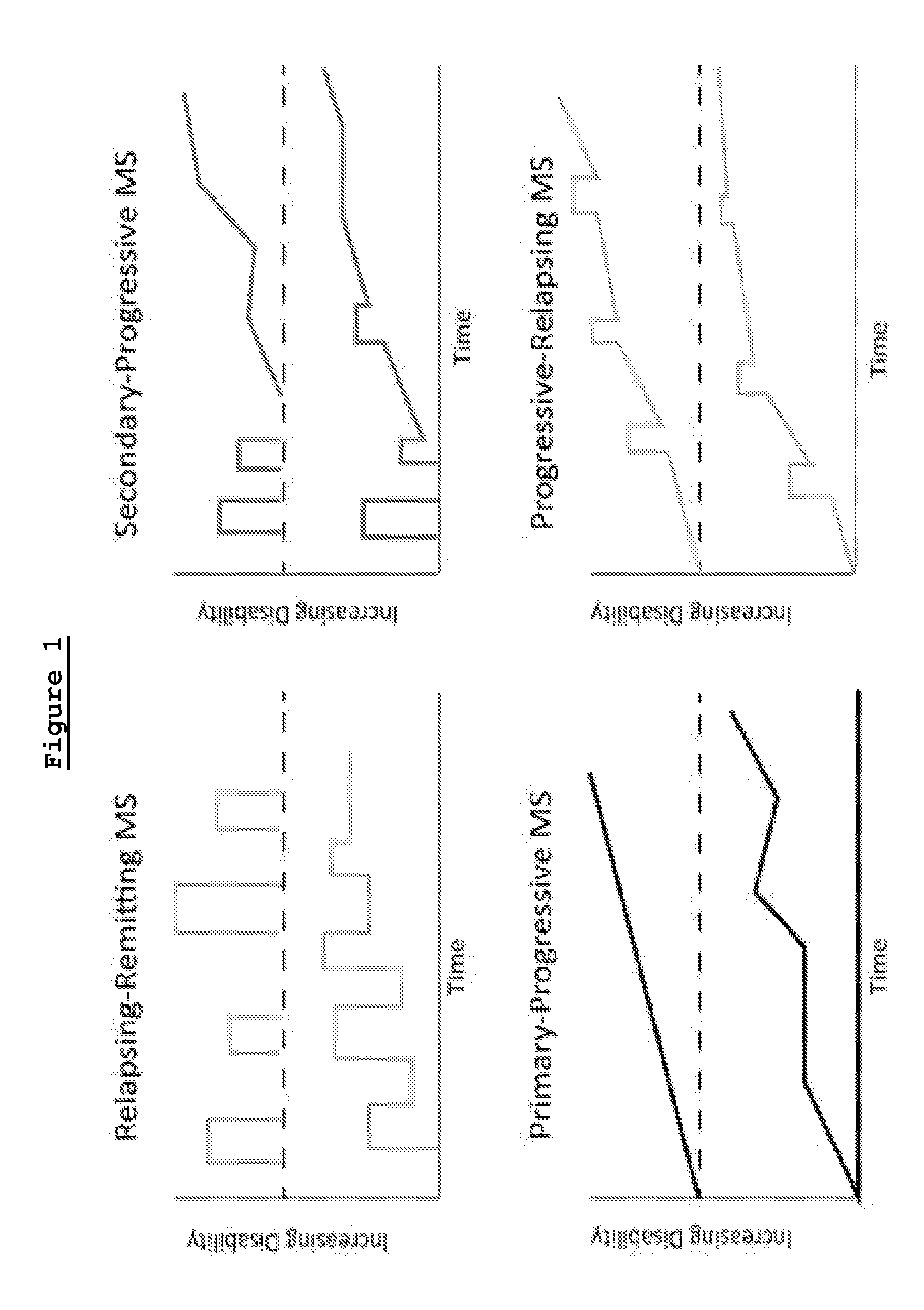
Treatment of progressive forms of multiple sclerosis with laquinimod
This invention provides a method for treating a human subject afflicted with a progressive form of multiple sclerosis, comprising periodically administering to the human subject an amount of laquinimod effective to treat the human subject. This invention also provides laquinimod for use in treating a human subject afflicted with a progressive form of multiple sclerosis. This invention further provides pharmaceutical compositions and packages comprising an effective amount of laquinimod for treating a progressive form of multiple sclerosis.
Owner:TEVA PHARMA IND LTD
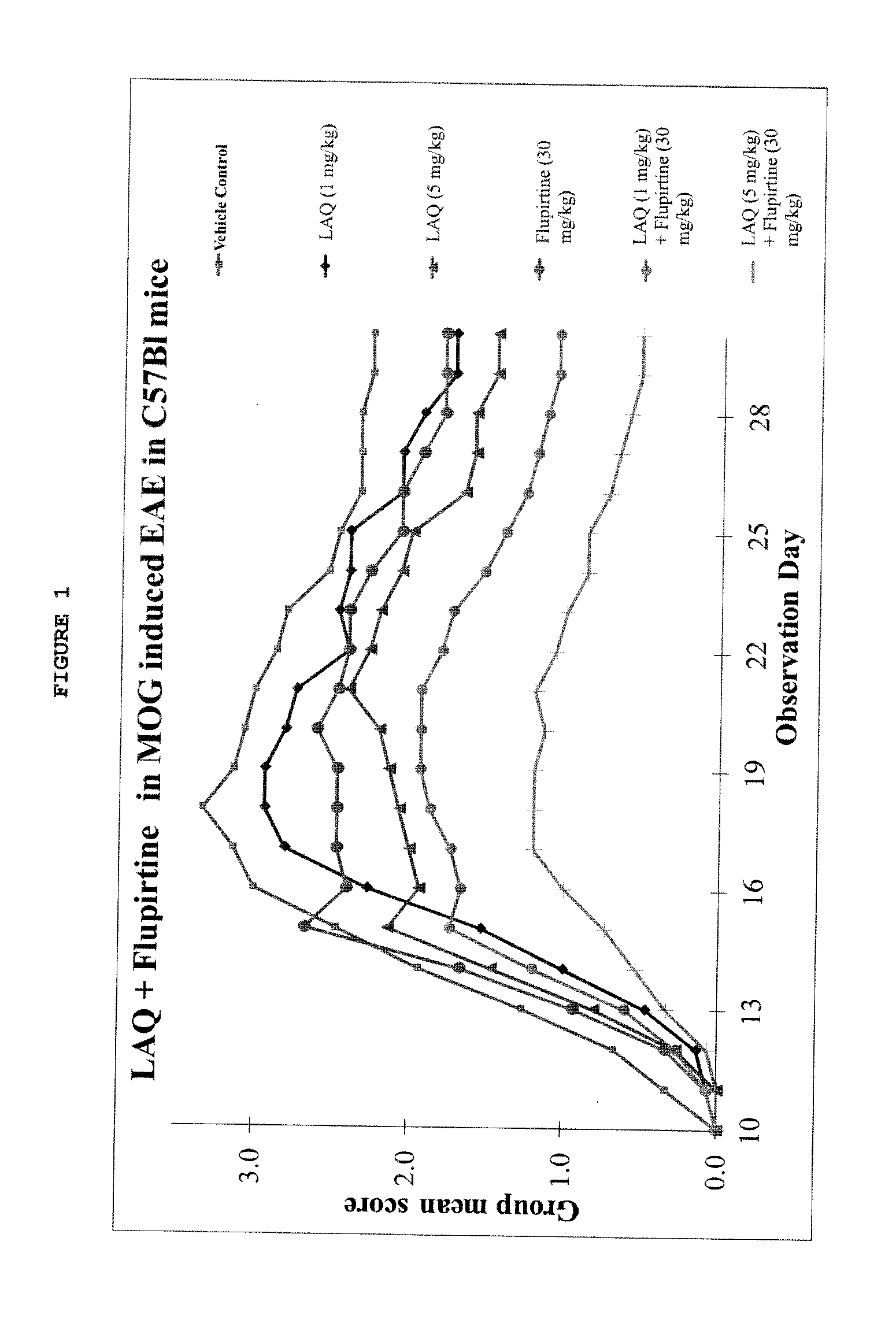
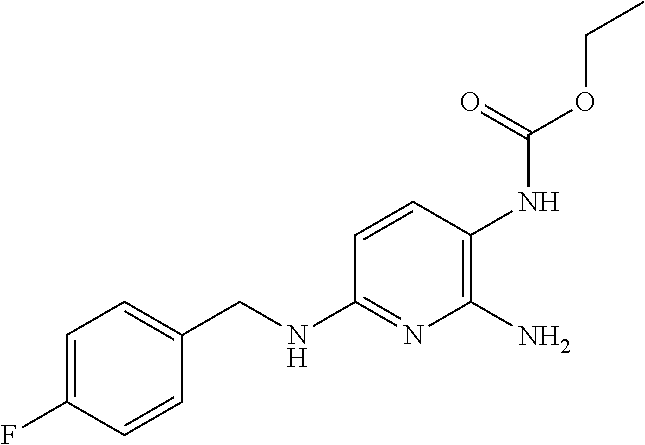
Treatment of multiple sclerosis with combination of laquinimod and flupirtine
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with flupirtine. This invention also provides a package and a pharmaceutical composition comprising laquinimod and flupirtine for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with flupirtine in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and flupirtine in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
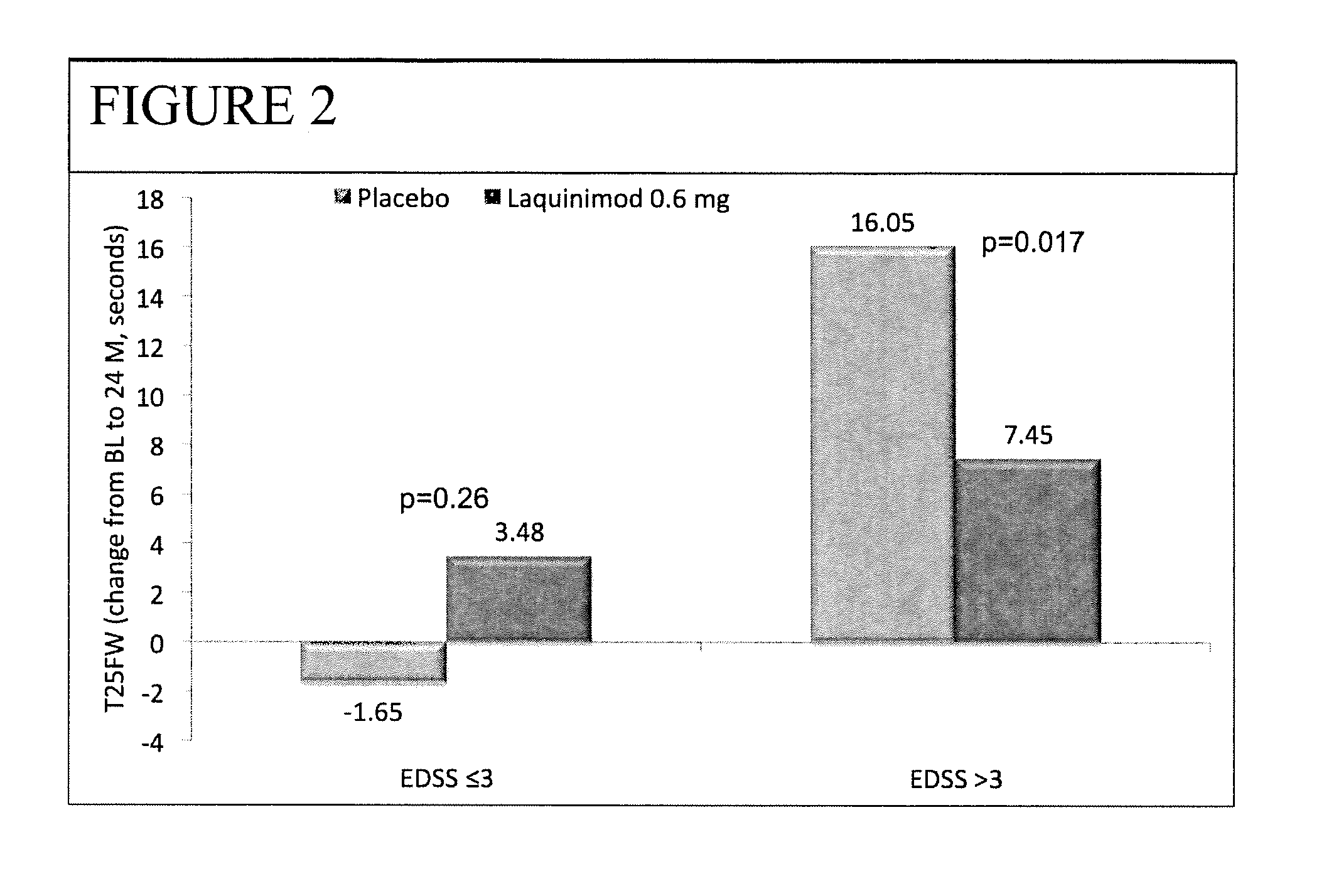
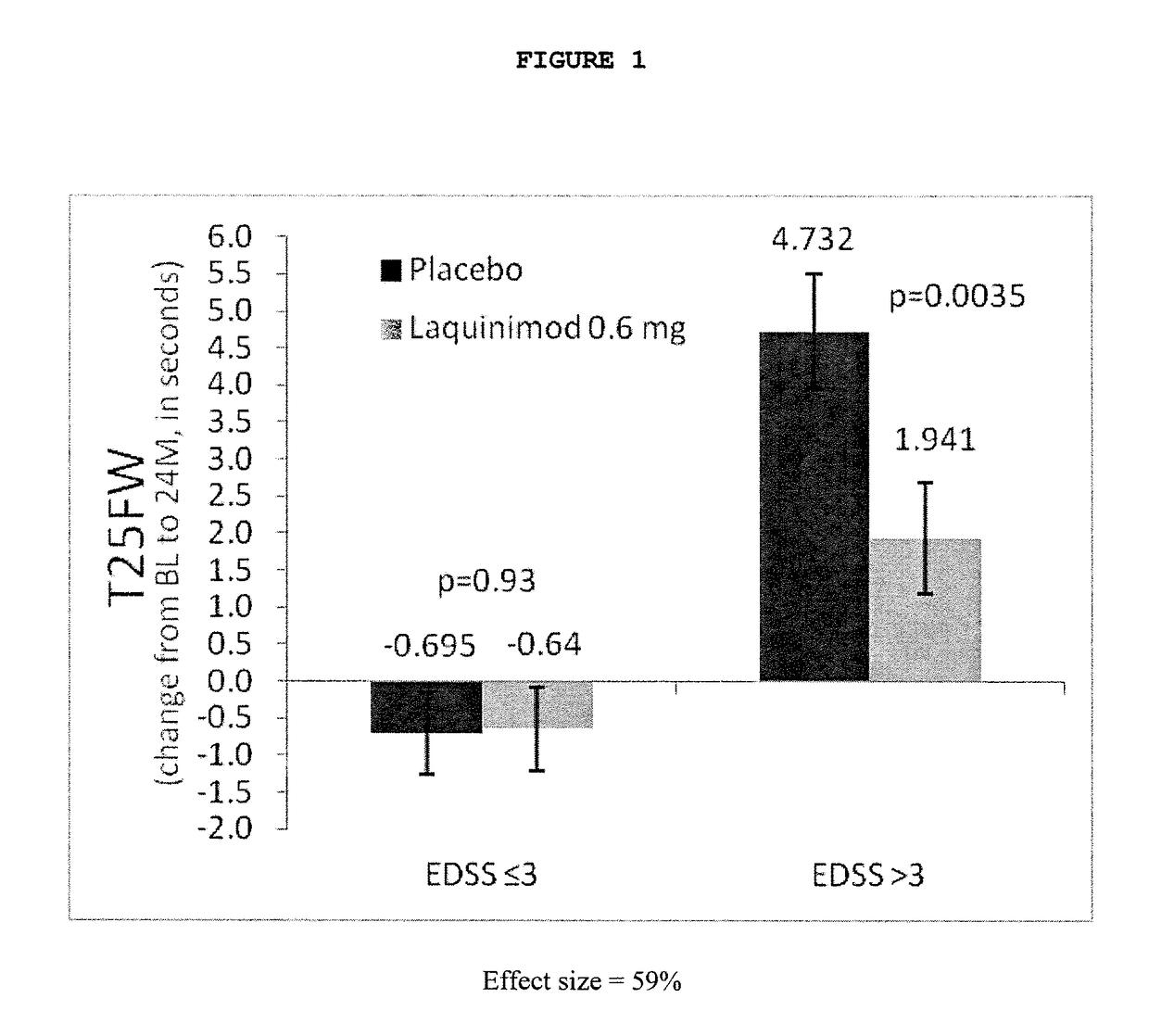
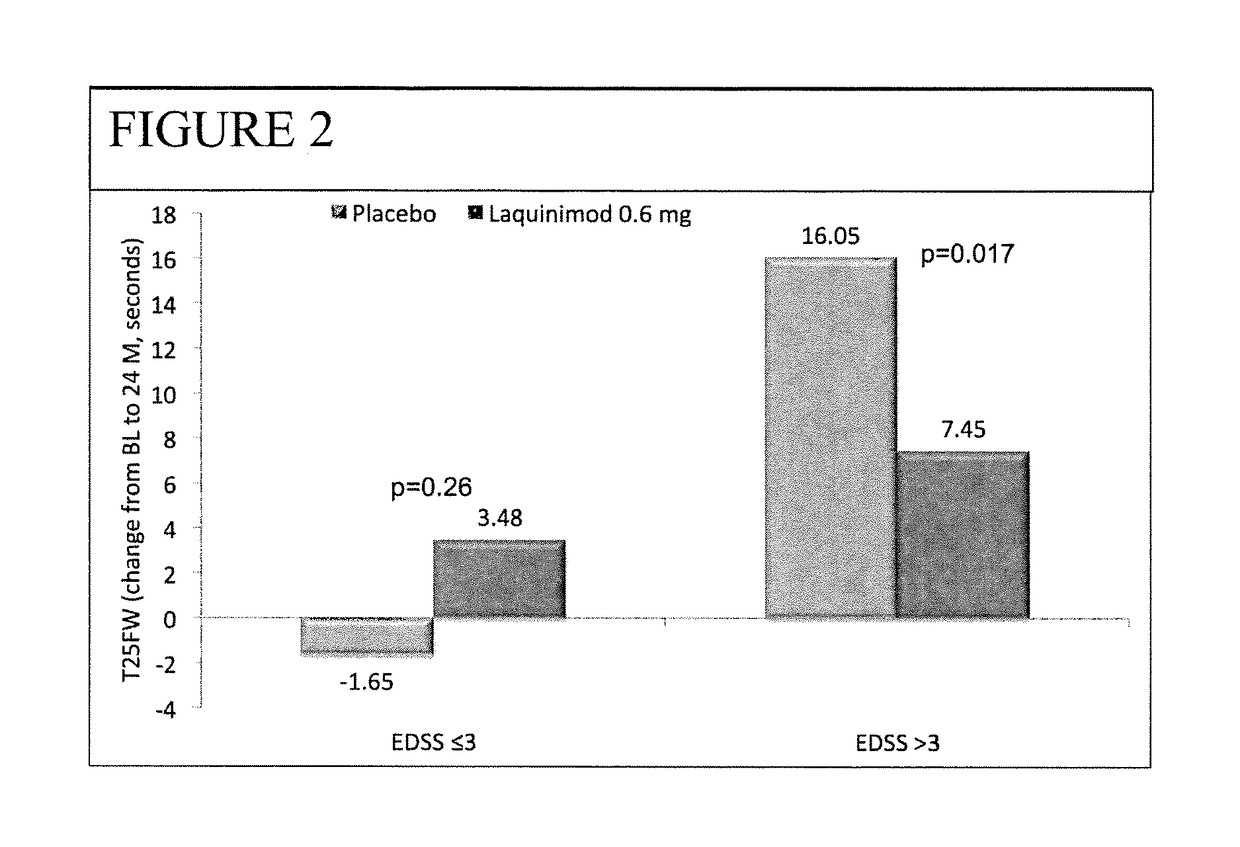
Laquinimod for the treatment of relapsing-remitting multiple sclerosis (RRMS) patients with a high disability status
InactiveUS20150306088A1Reducing ambulatory deteriorationReduce ambulatory deteriorationBiocideNervous disorderLaquinimodHuman patient
This invention provides a method for treating or for reducing ambulatory deterioration in a human patient diagnosed to be afflicted with relapsing-remitting multiple sclerosis (RRMS) and having a high baseline disability score according to the Kurtzke Expanded Disability Status Scale (EDSS), comprising periodically administering to only the patient diagnosed with RRMS and having a high baseline disability score an amount of laquinimod effective to treat the patient or to reduce ambulatory deterioration. This invention further provides pharmaceutical compositions and packages comprising an effective amount of laquinimod for treating a human patient diagnosed to be afflicted with RRMS and having a high baseline disability score according to the EDSS.
Owner:TEVA PHARMA IND LTD
Use of high dose laquinimod for treating multiple sclerosis
InactiveUS20160000775A1Reducing brain atrophyExtension of timeBiocideNervous disorderAtrophyHigh doses
Disclosed herein are methods of treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome, methods for treating a human subject by providing neuroprotection to the human subject, and methods of treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome by increasing the time to confirmed disease progression, increasing the time to confirmed relapse or reducing brain atrophy in the human patient, comprising orally administering to the human patient or subject a daily dose of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof. The subject invention also provides a pharmaceutical oral unit dosage form of about 1.2 mg laquinimod or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier for use in treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome, for use in treating a human subject by providing neuroprotection to the human subject, or for use treating a human patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome by increasing the time to confirmed disease progression, increasing the time to confirmed relapse or reducing brain atrophy in the human patient.
Owner:TEVA PHARMA IND LTD
Laquinimod for the treatment of relapsing-remitting multiple sclerosis (RRMS) patients with a high disability status
InactiveUS9662322B2Reducing ambulatory deteriorationHigh baseline disability scoreNervous disorderPeptide/protein ingredientsLaquinimodHuman patient
This invention provides a method for treating or for reducing ambulatory deterioration in a human patient diagnosed to be afflicted with relapsing-remitting multiple sclerosis (RRMS) and having a high baseline disability score according to the Kurtzke Expanded Disability Status Scale (EDSS), comprising periodically administering to only the patient diagnosed with RRMS and having a high baseline disability score an amount of laquinimod effective to treat the patient or to reduce ambulatory deterioration. This invention further provides pharmaceutical compositions and packages comprising an effective amount of laquinimod for treating a human patient diagnosed to be afflicted with RRMS and having a high baseline disability score according to the EDSS.
Owner:TEVA PHARMA IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com