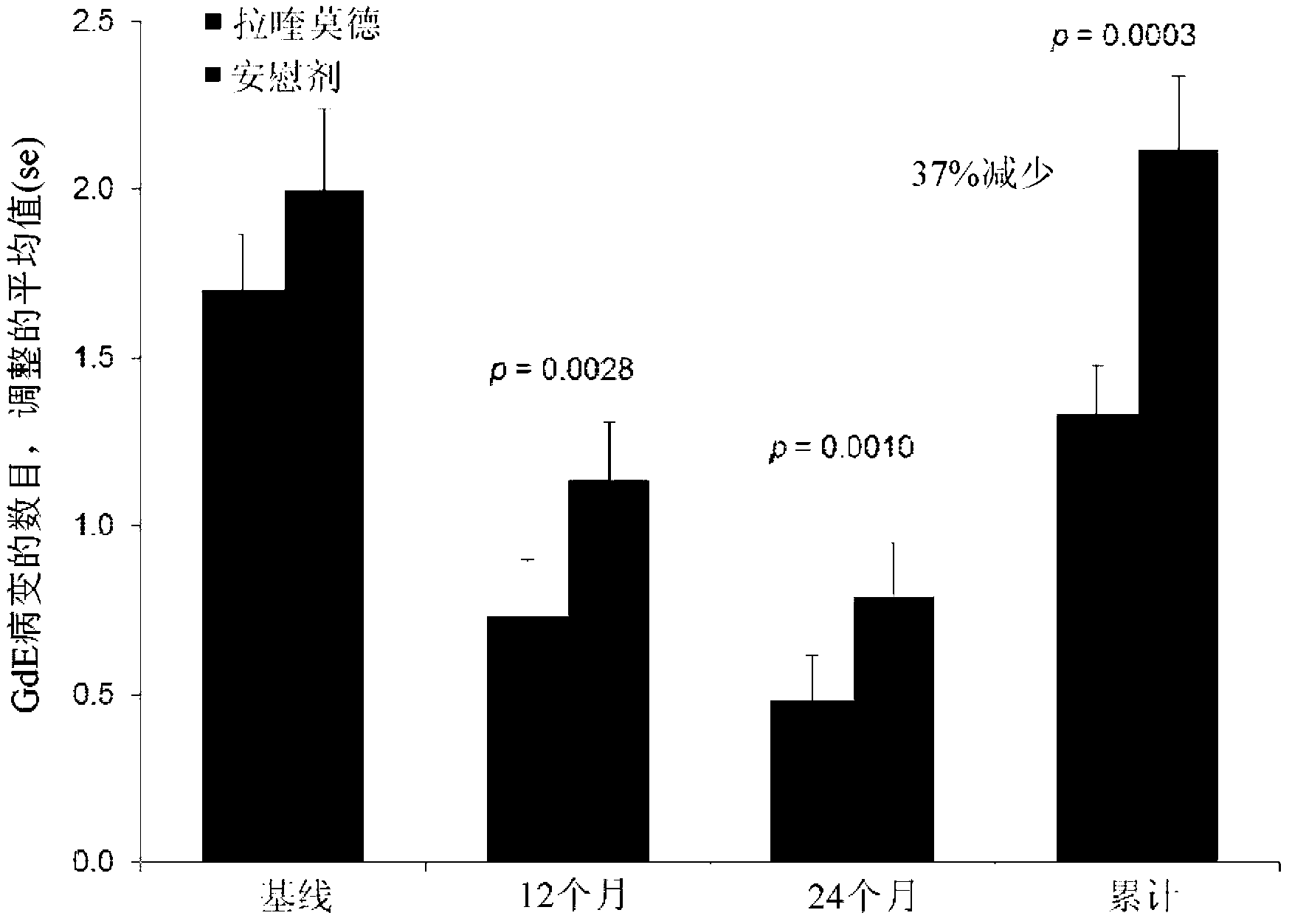Use of laquinimod for reducing fatigue, improving functional status, and improving quality of life in multiple sclerosis patients
A technology for multiple sclerosis, functional status, in the field of use of laquinimod to reduce fatigue, improve functional status and improve quality of life in multiple sclerosis patients, addressing unidentified issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
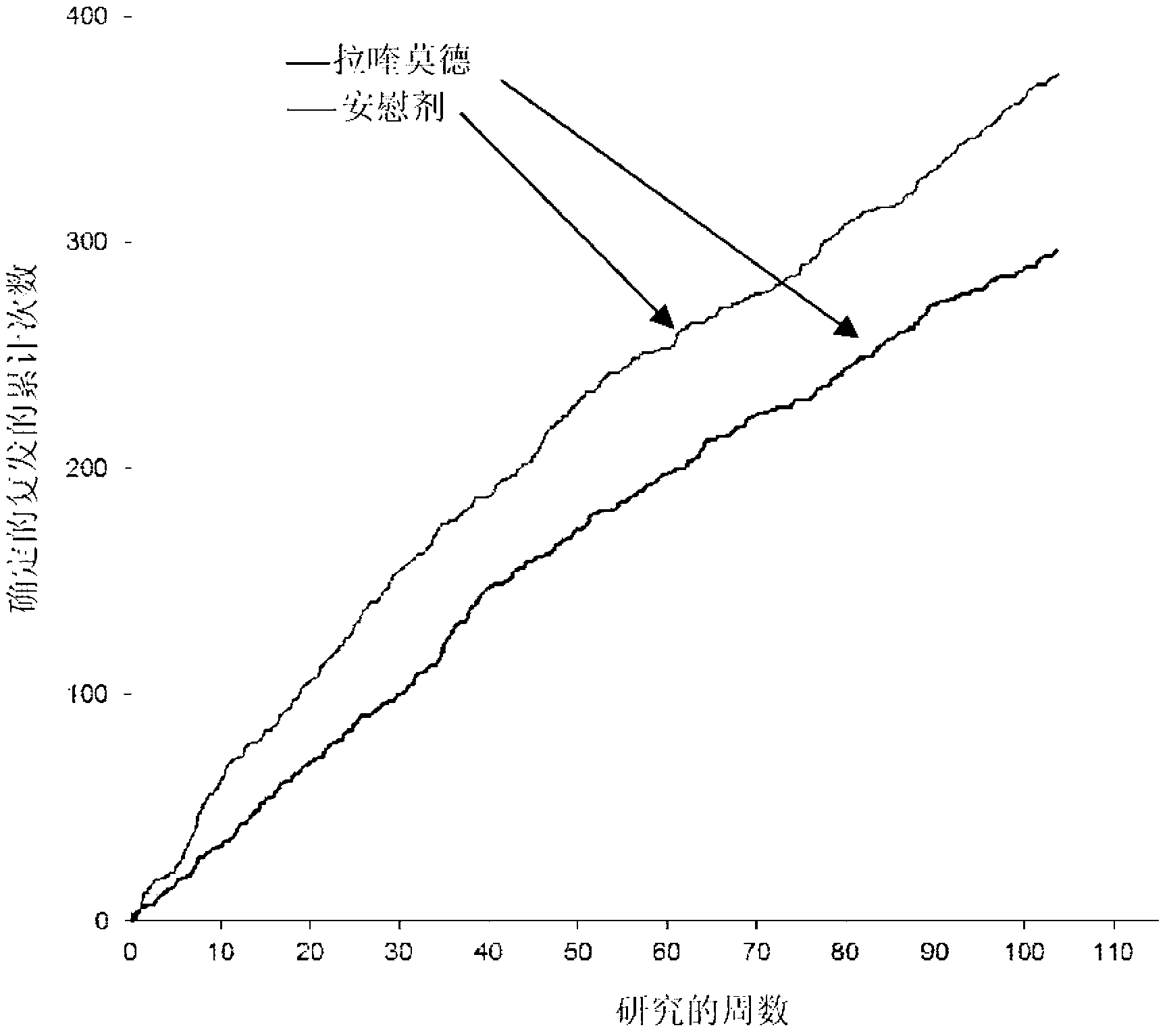
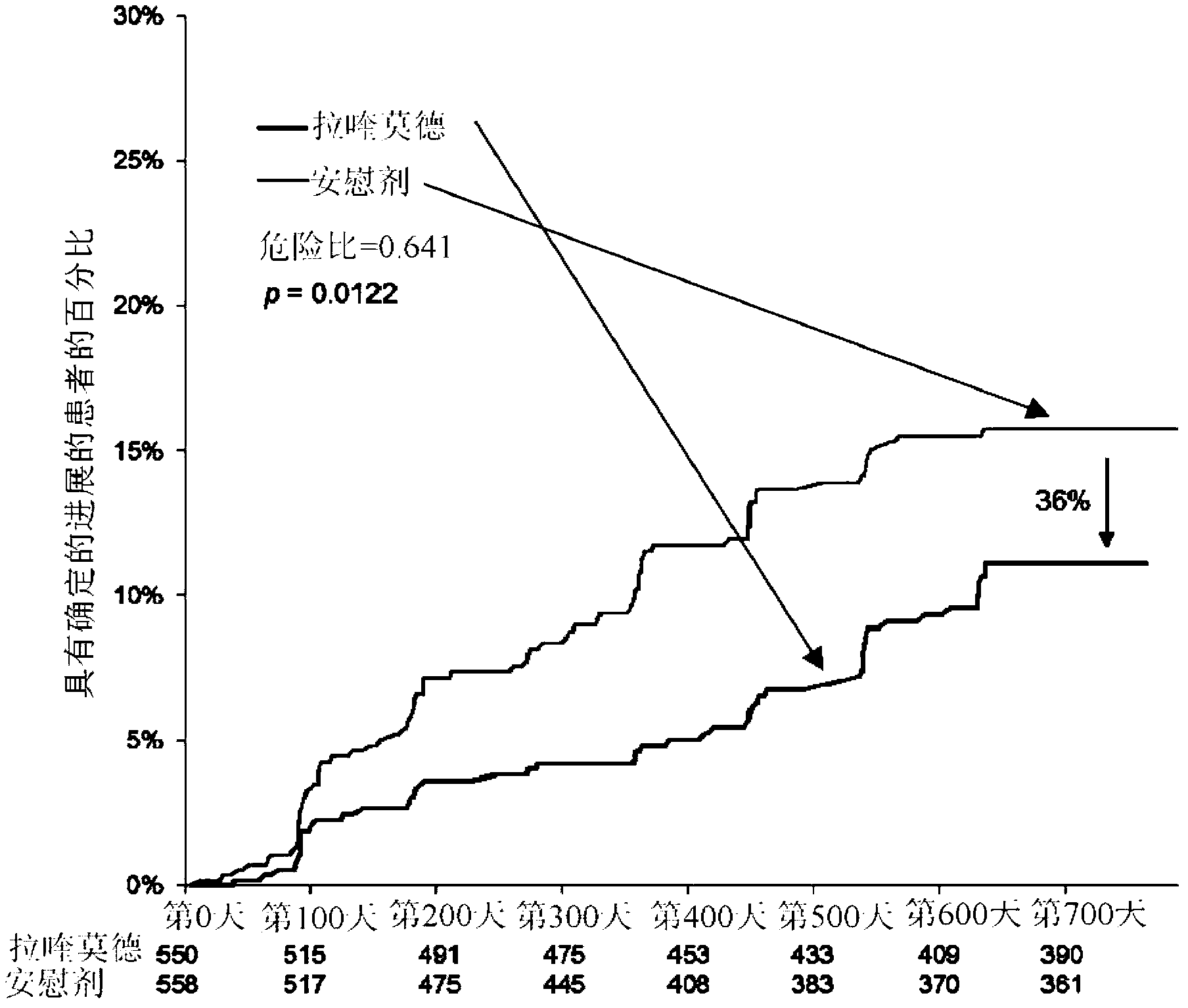
[0100] Example 1: Clinical Trial (Phase III) - Evaluation of Oral Laquinimod in Prevention of MS Progression
[0101] Conducted a multinational (24 countries), multicenter (approximately 139 sites), randomized, double-blind, parallel-group, placebo-controlled clinical trial ("ALLEGRO" or MS-LAQ-301) to evaluate Efficacy, safety and tolerability of daily oral administration of 0.6 mg laquinimod for a period of 24 months in individuals with recurrent multiple sclerosis (RRMS).
[0102] One thousand one hundred and six (1106) patients were randomized equally to 0.6 mg laquinimod or placebo and treated in a double-blind fashion with baseline characteristics balanced between the groups. The primary endpoint of the study was the number of relapses identified during the double-blind treatment period, which corresponds to the annualized relapse rate (ARR - number of relapses divided by total exposure for all patients). Secondary endpoints included disability as measured by change i...
example 2
[0306] Example 2: Clinical Trial (Phase III) - and laquinimod benefit-risk assessment
[0307] A multinational, multicenter, randomized, parallel group clinical trial ("BRAVO") was conducted in individuals with RRMS. BRAVO was conducted in a double-blind and rater-blinded design to evaluate laquinimod versus placebo and interferon beta-1a Efficacy, safety and tolerability of the reference group. The study also aimed to administer oral laquinimod versus injectable interferon beta-1a Comparative benefit / risk assessments between.
[0308] The primary objective of this study was to assess the efficacy of laquinimod at a daily dose of 0.6 mg in individuals with RRMS by measuring the number of relapses determined during the treatment period. Secondary objectives of this study included assessing the effect of laquinimod at a daily dose of 0.6 mg on disability accumulation by assessing MSFC scores at the end of the treatment period; To assess the effect of laquinimod at a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com