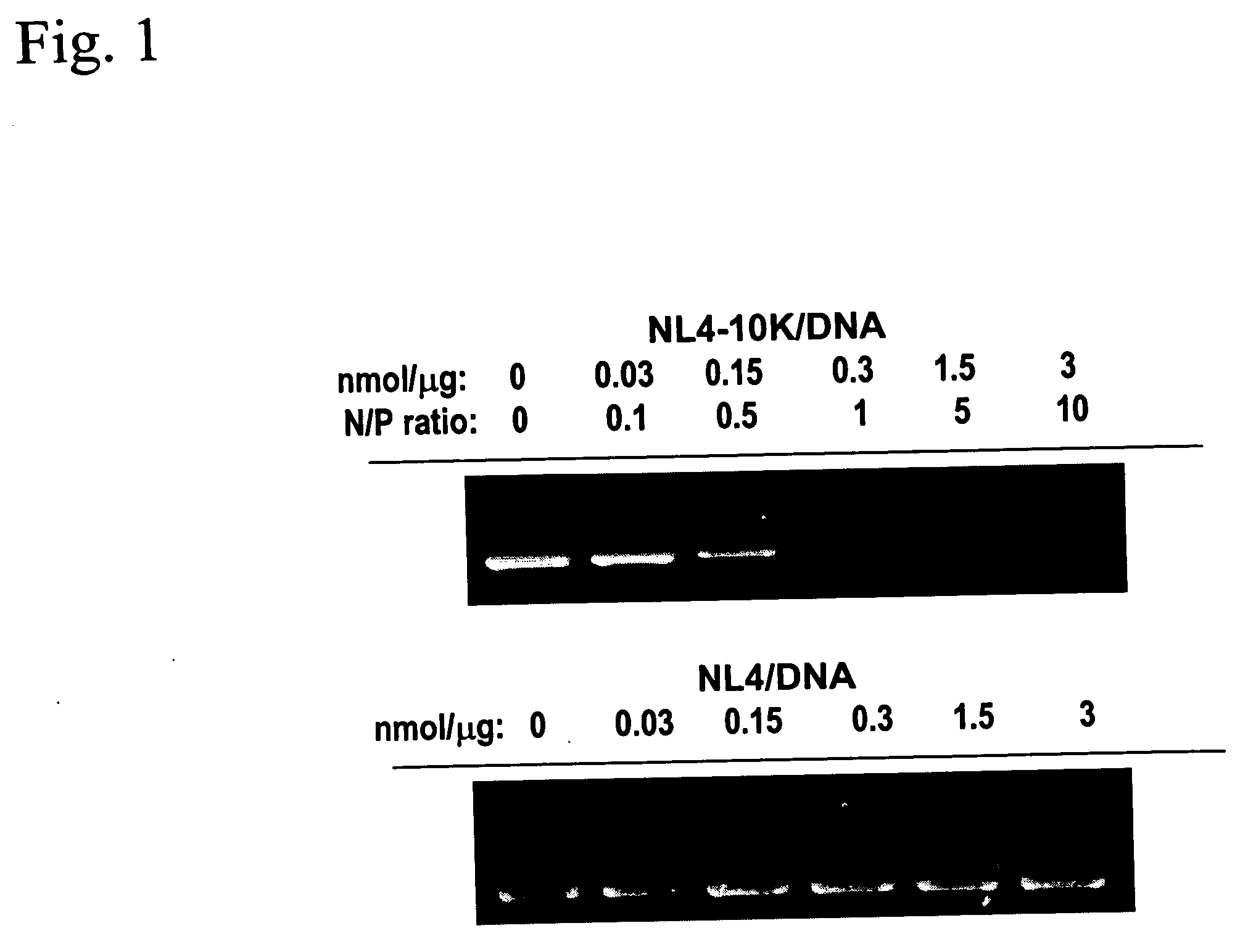
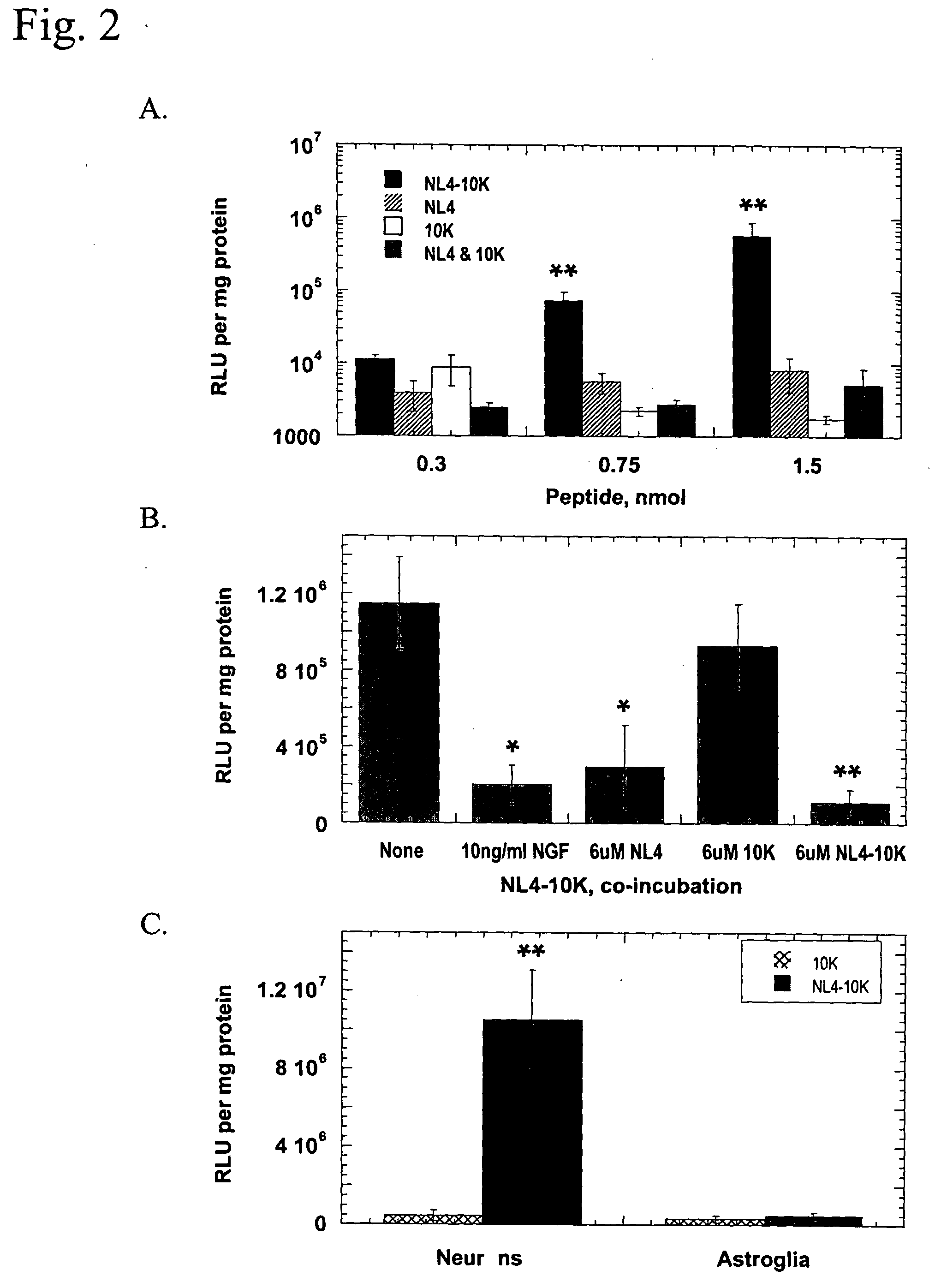
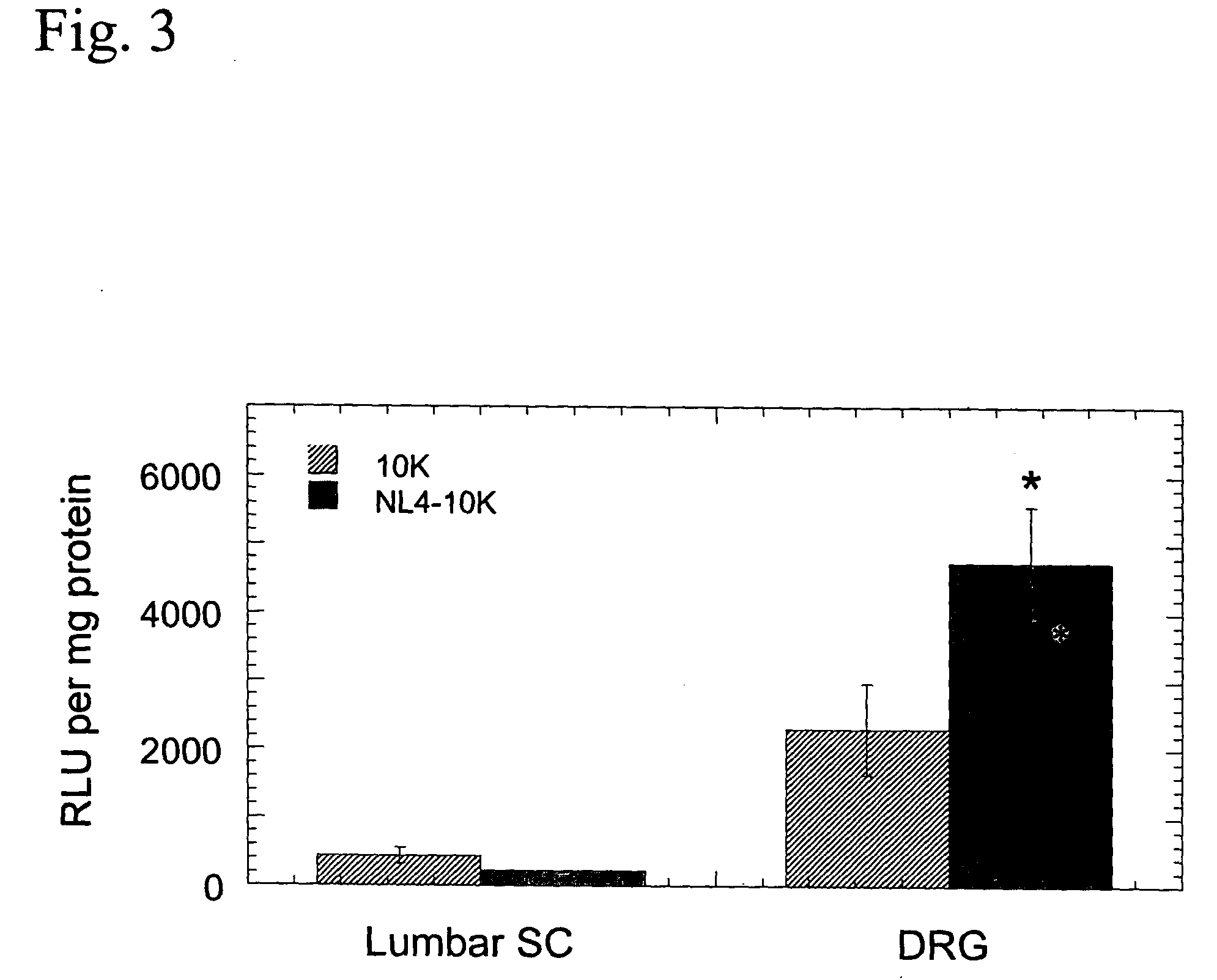
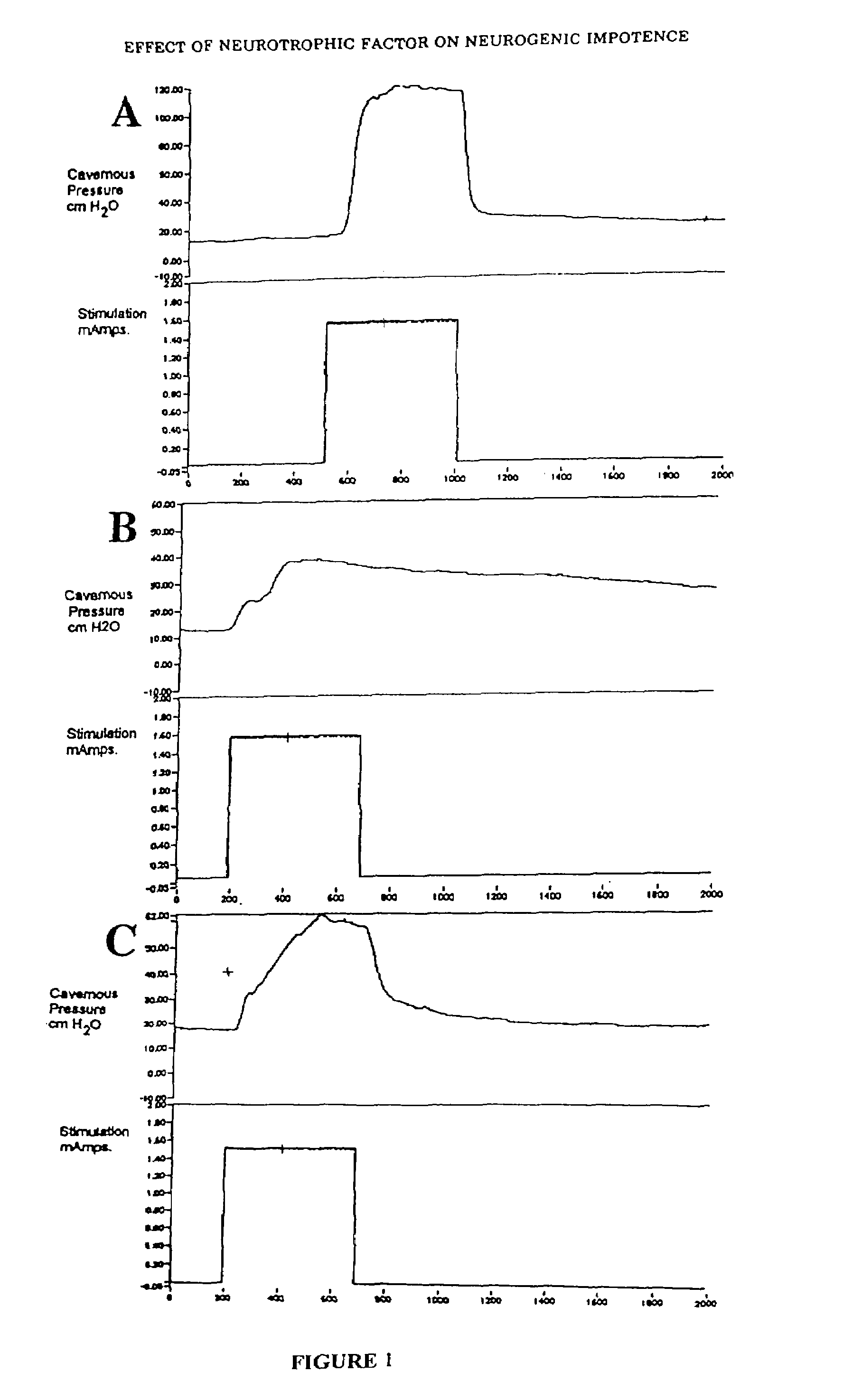
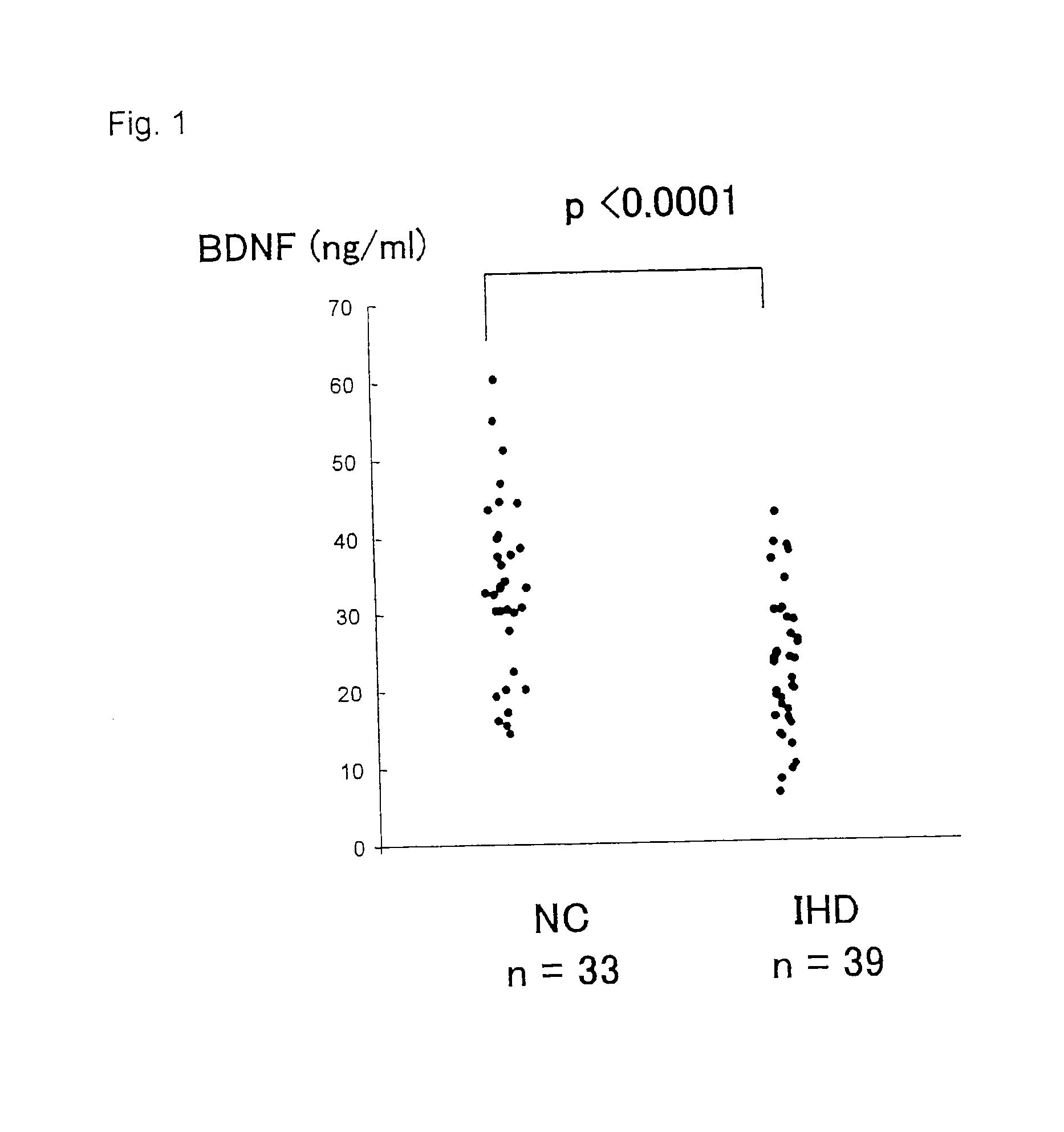Patents
Literature
130 results about "Brain-derived growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
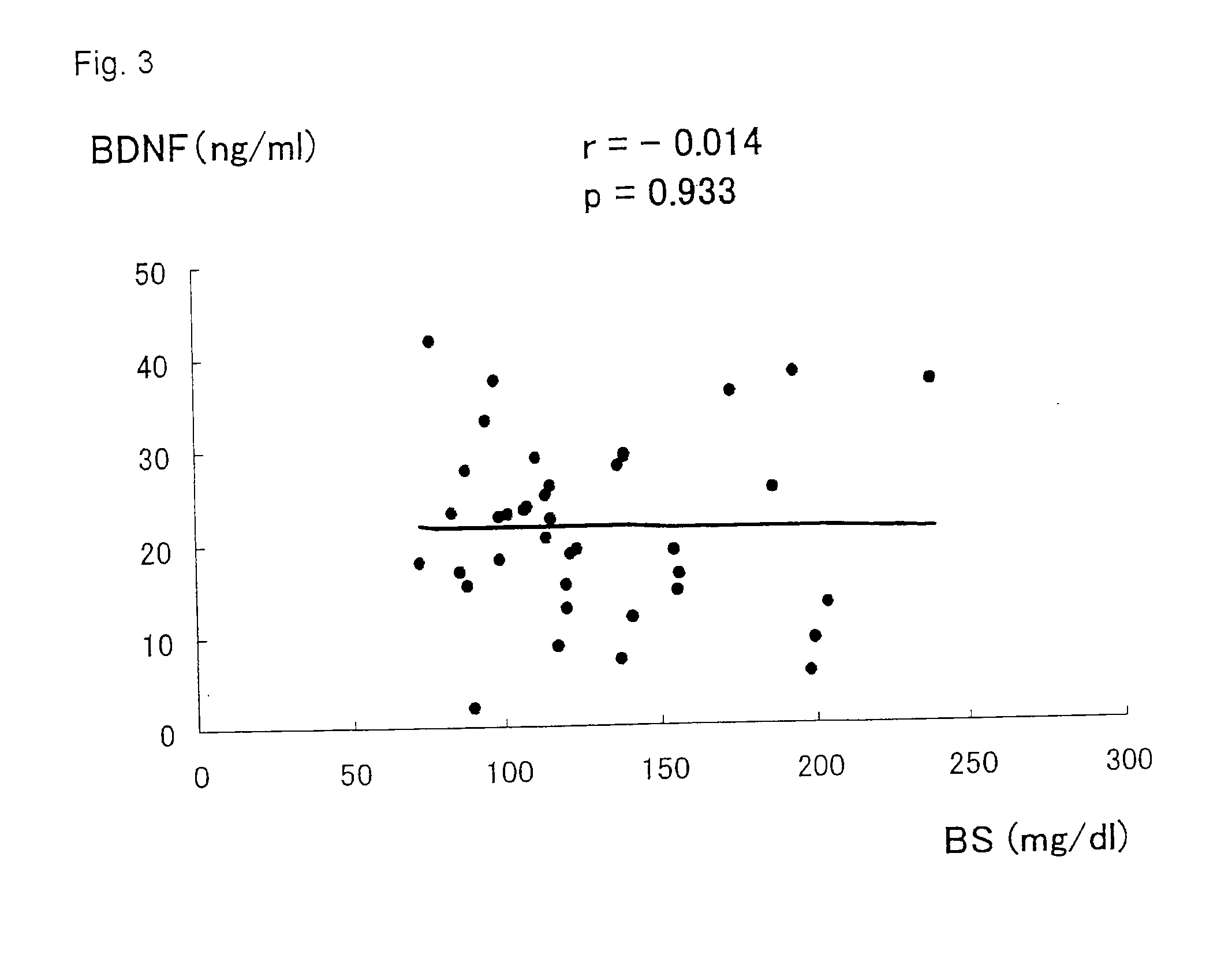
Brain-derived neurotrophic factor, also known as BDNF, is a protein that, in humans, is encoded by the BDNF gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the canonical Nerve Growth Factor.
Method and system for modulating energy expenditure and neurotrophic factors
InactiveUS20080046012A1Symptoms improvedImprove performanceElectrotherapyImplantable ElectrodesRegimen
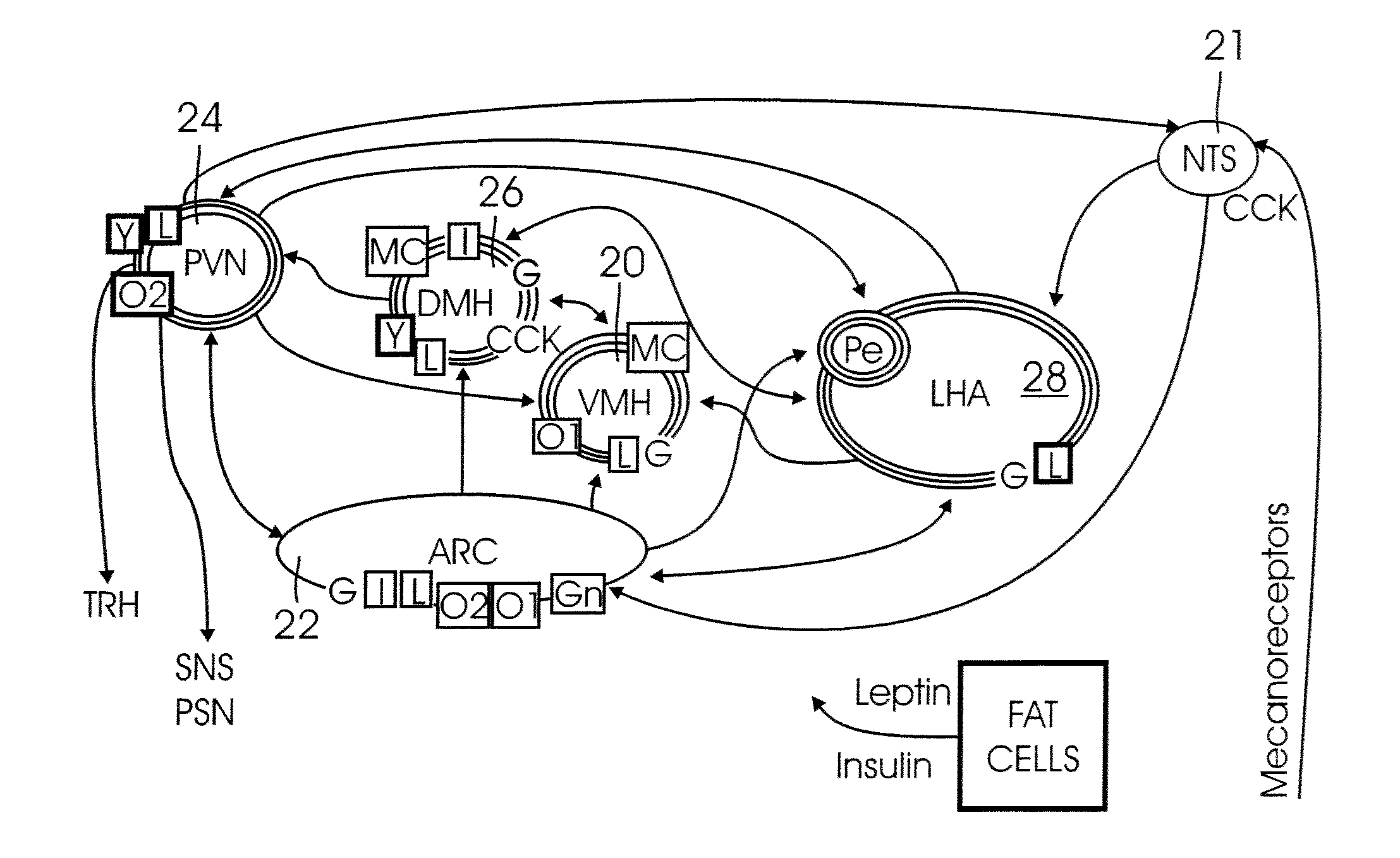
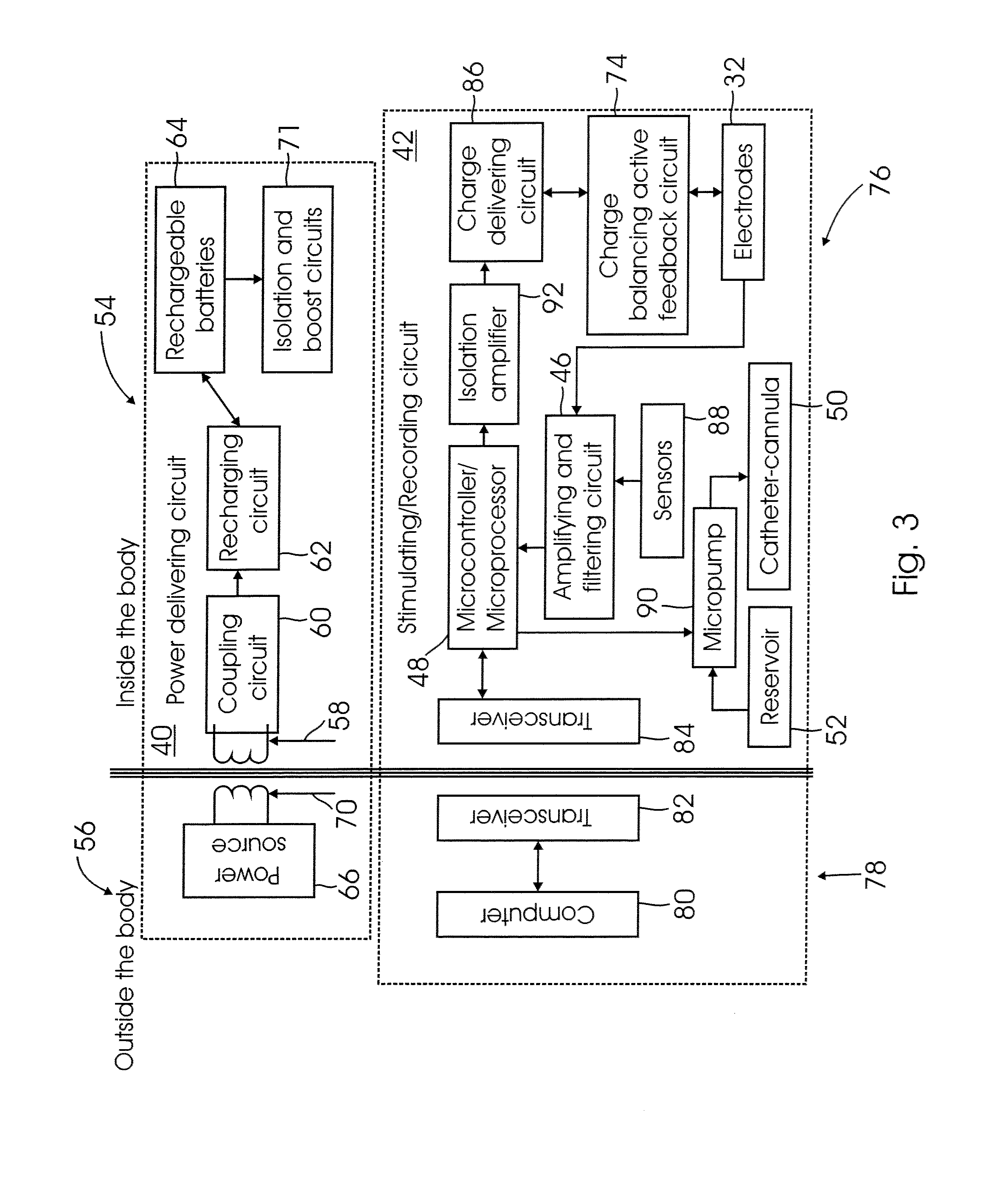
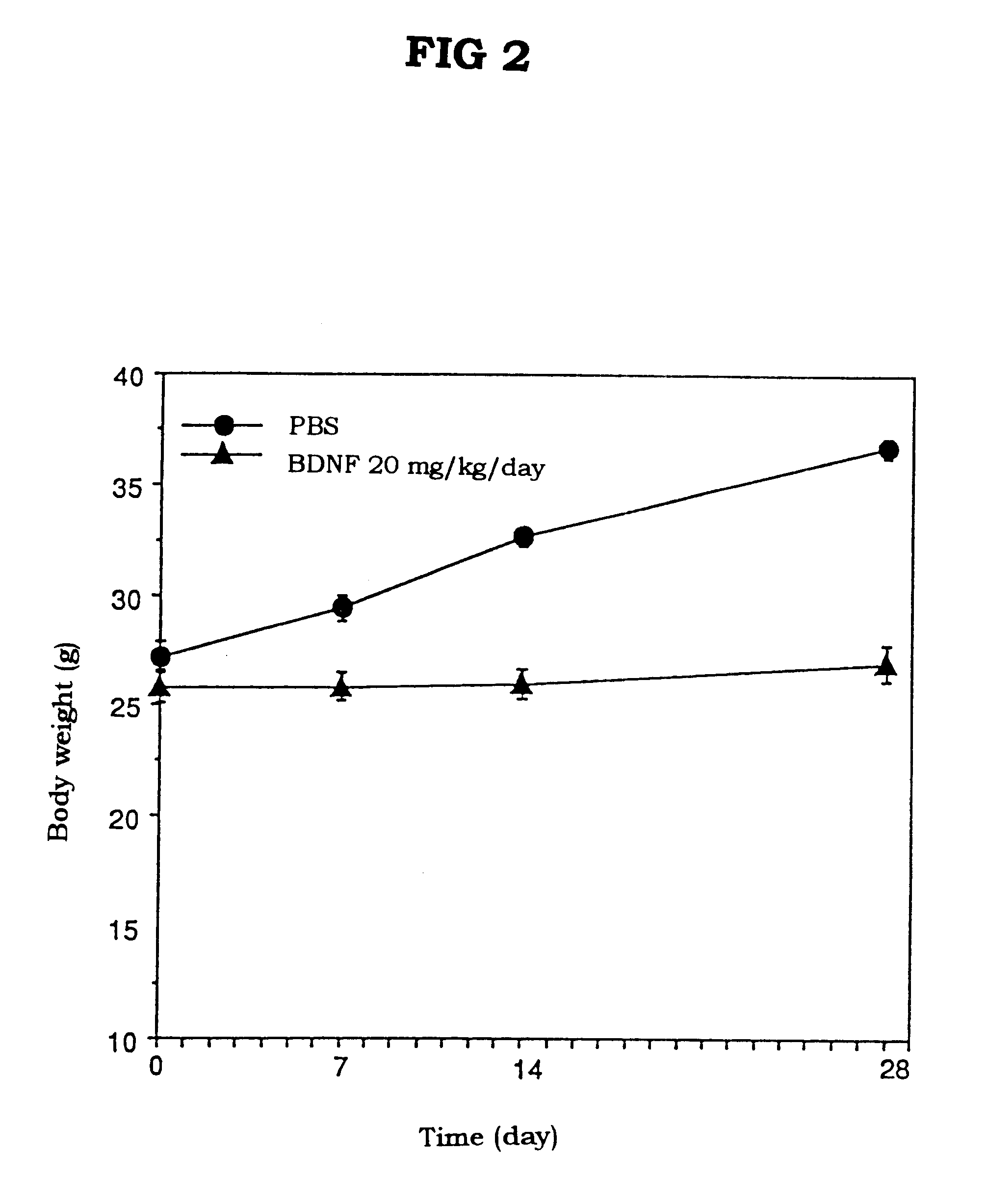
A method system for modulating the energy expenditure and / or the expressed brain-derived neurotrophic factor (BDNF) in the brain of an individual is performed by a system that includes a control device that generates a stimulation pattern from a predetermined set of stimulation parameters, and that converts the stimulation pattern into a stimulation signal. A stimulation signal delivery mechanism, configured for implantation into a selected part of the brain, receives the stimulation signal from the control device and delivers the signal to the selected part of the brain. The stimulation signal may be an electrical signal delivered by a brain-implantable electrode, or a chemical signal in the form of a drug dosage regimen delivered by an implantable micropump under the control of the control device. Modulation of the energy expenditure and / or BDNF is achieved by the stimulation of the hypothalamus, either directly or indirectly, by the stimulation signal.
Owner:RGT UNIV OF CALIFORNIA
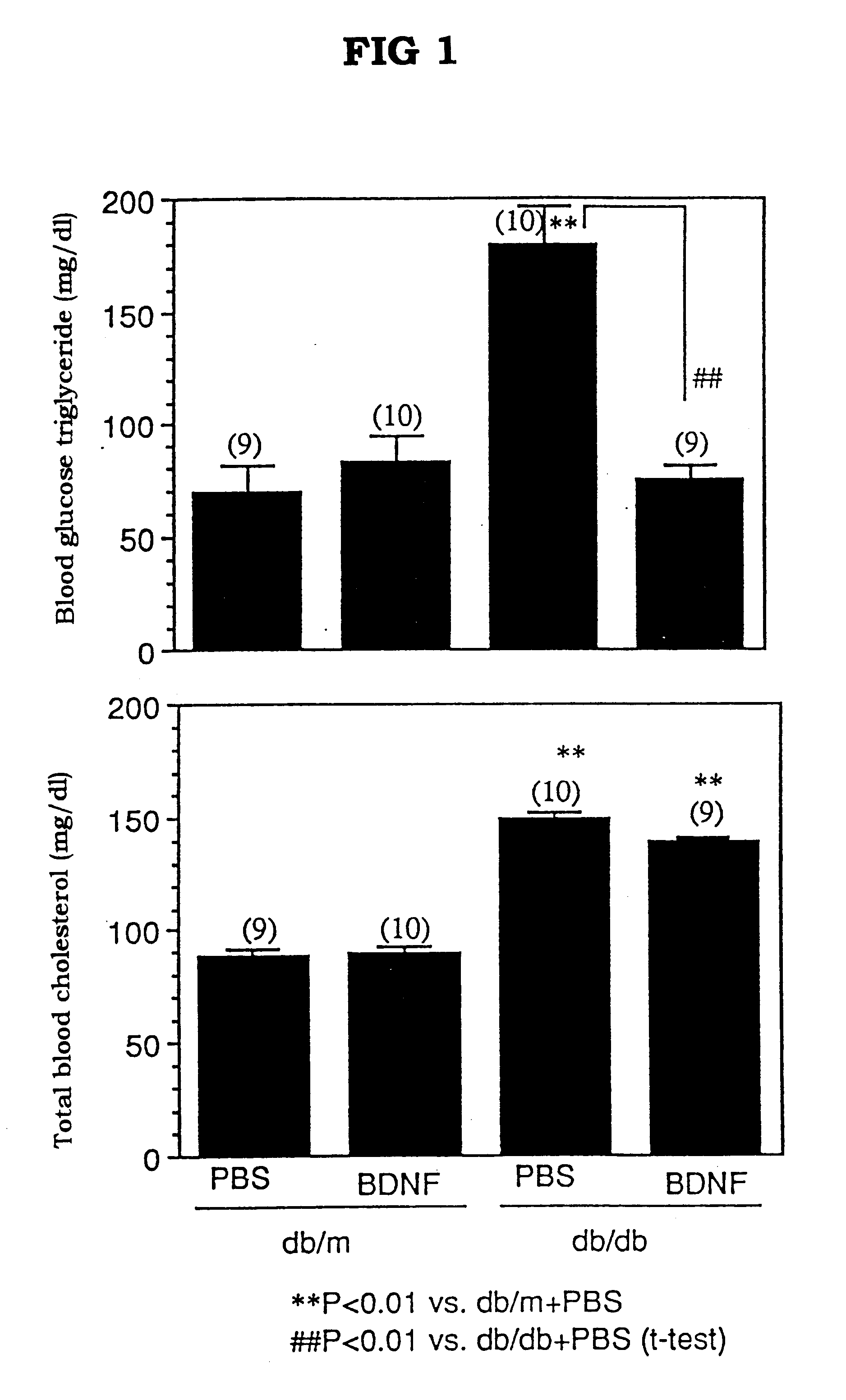
Hepatocyte growth factor for treatment of diabetes
InactiveUS6472366B2Lower blood sugar levelsLower Level RequirementsBiocidePeptide/protein ingredientsCvd riskBULK ACTIVE INGREDIENT
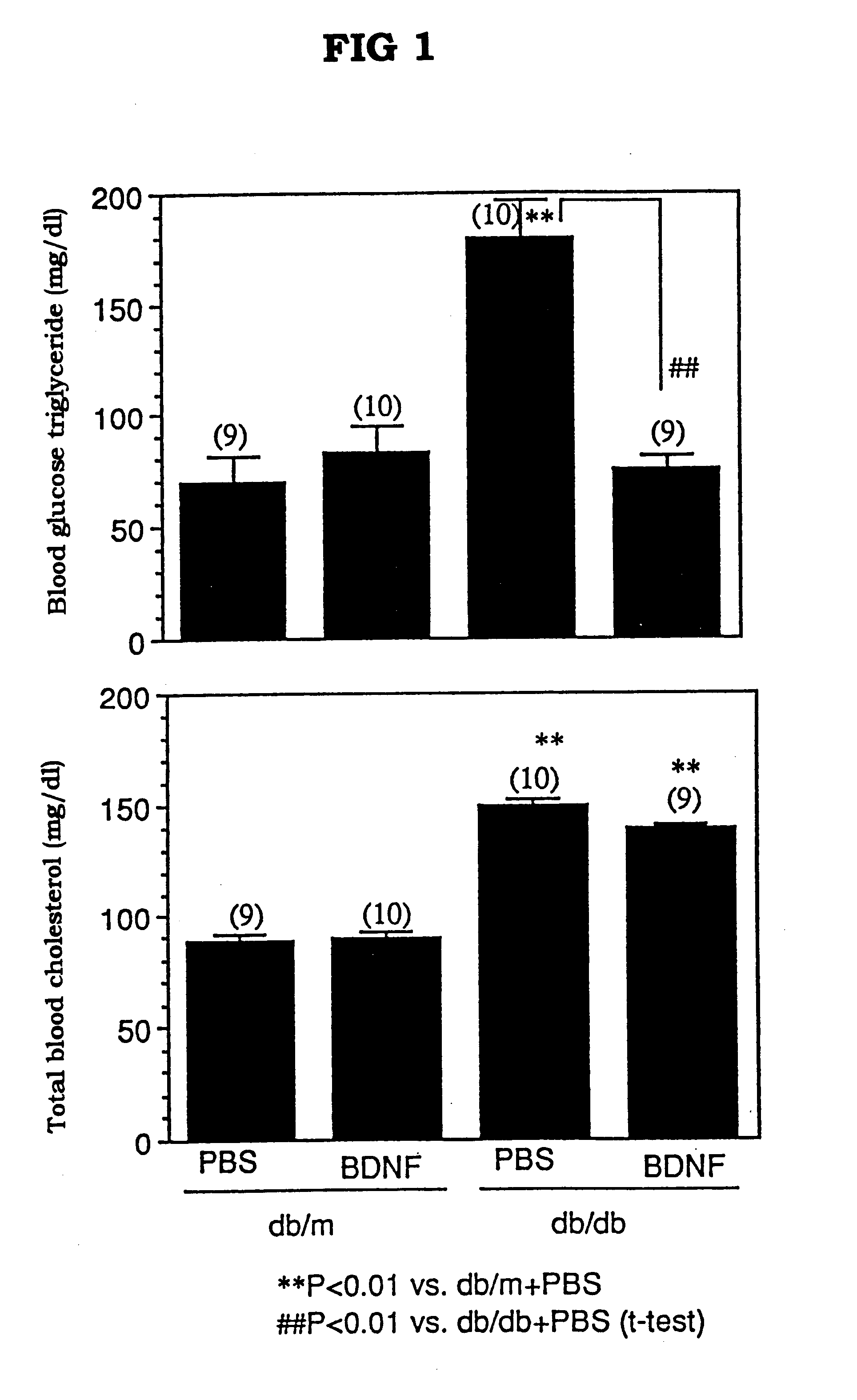
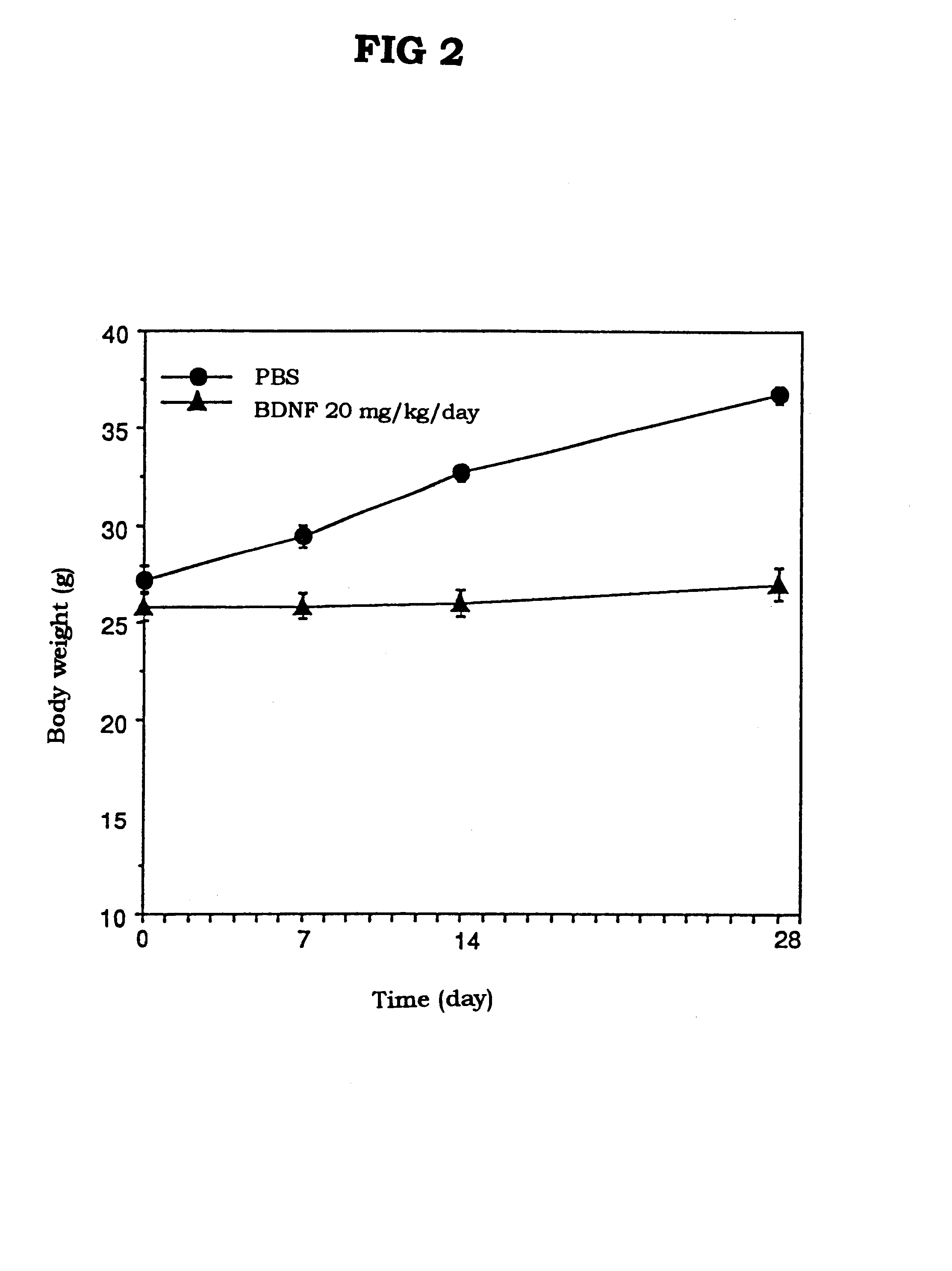
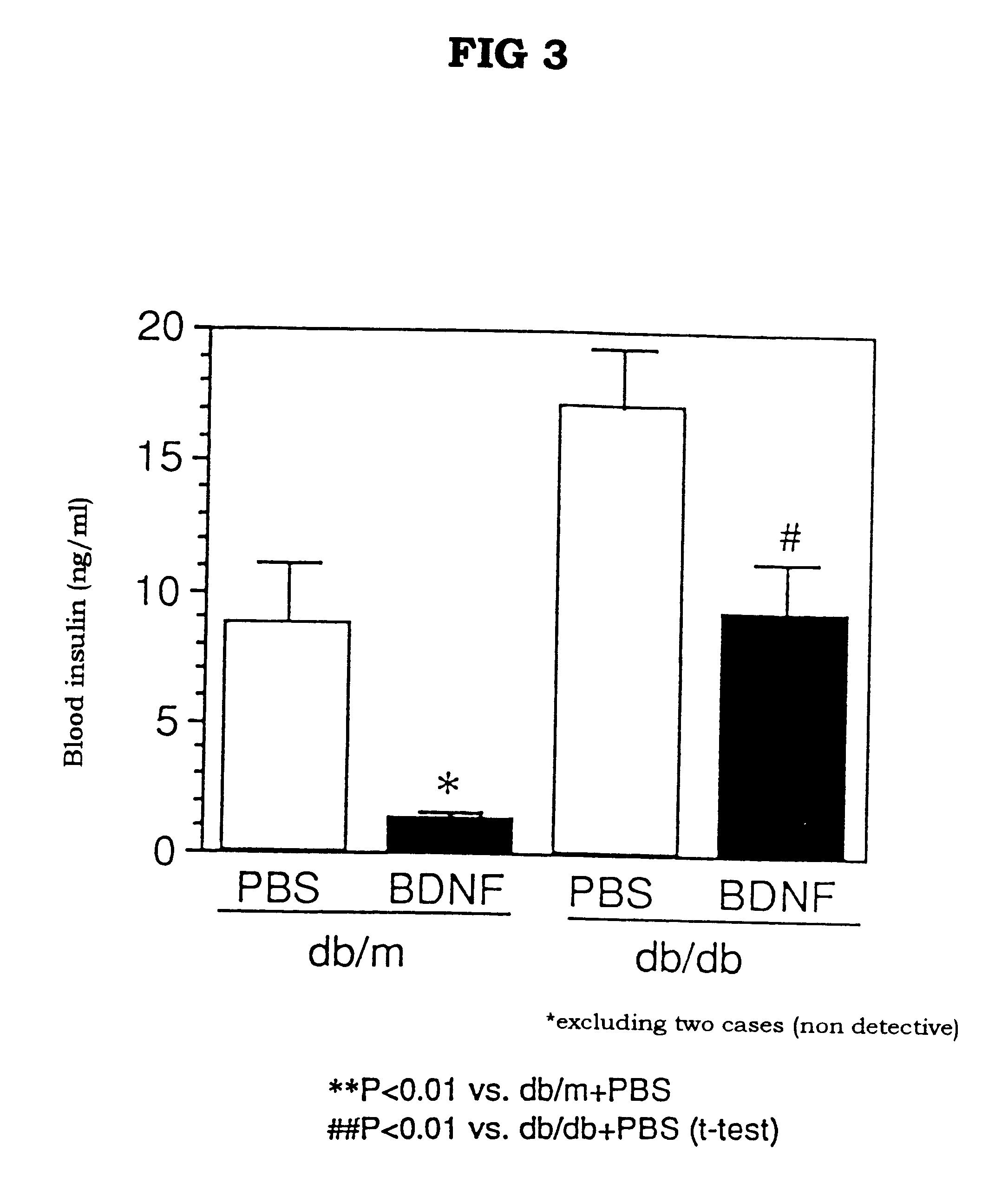
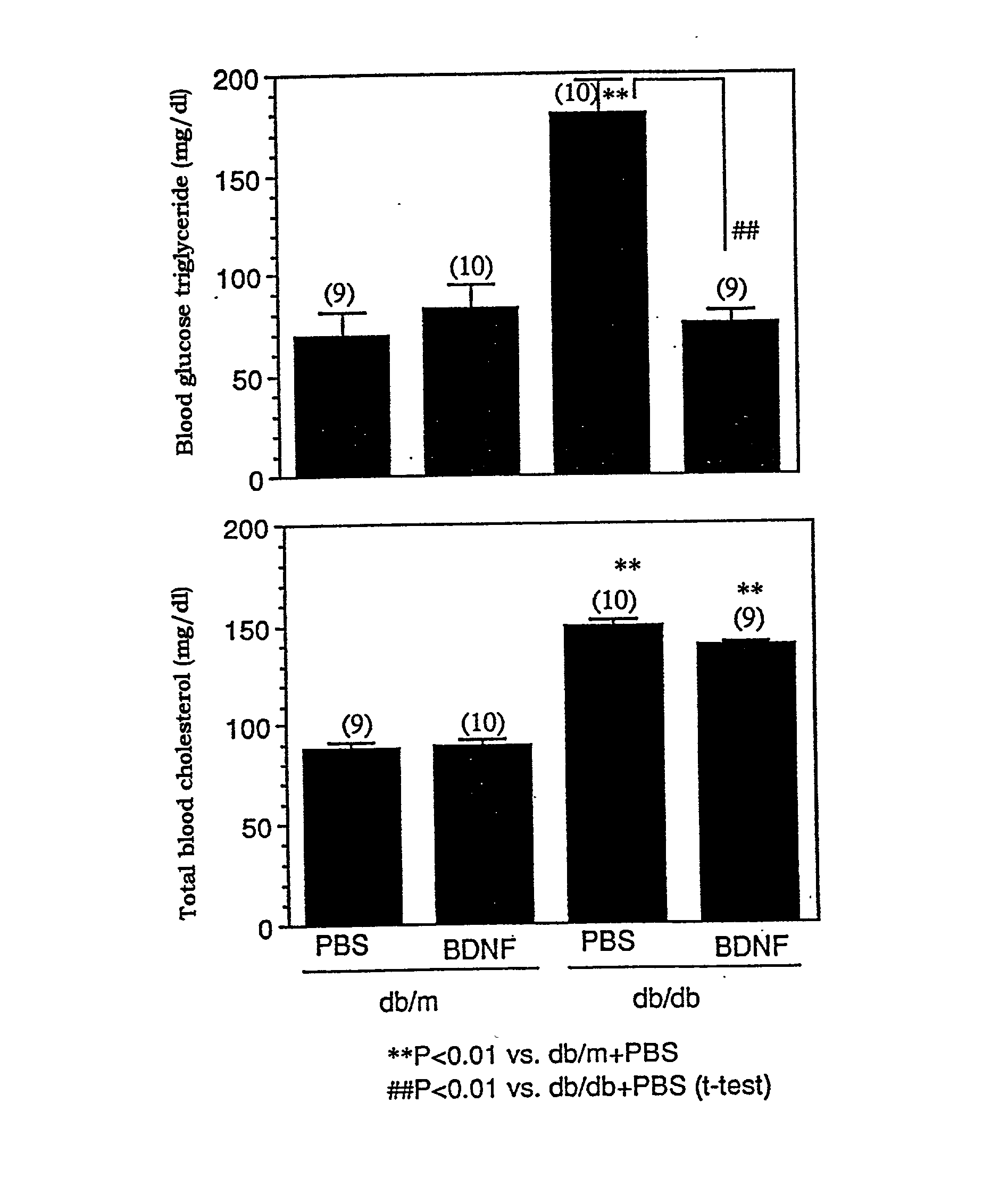
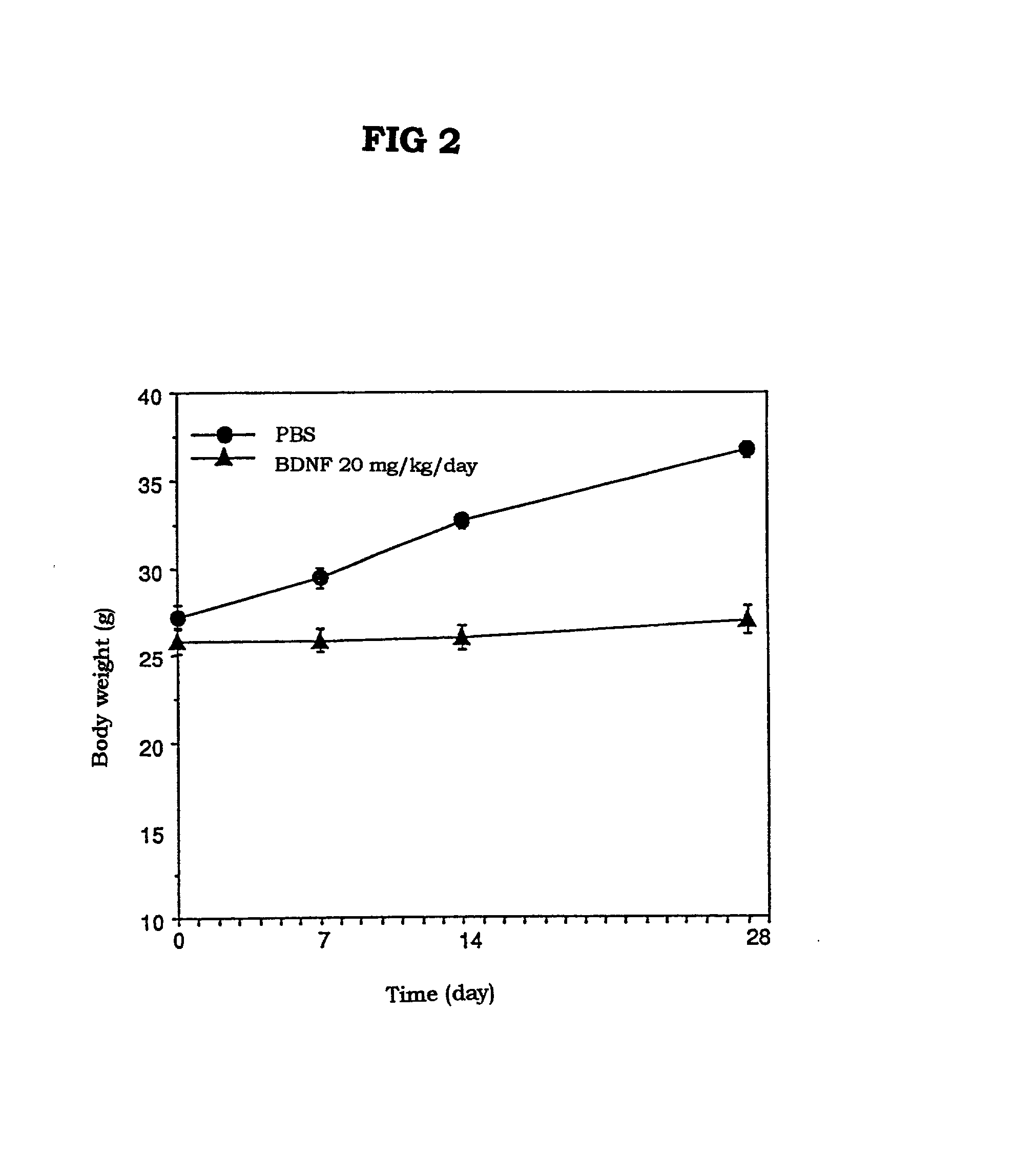
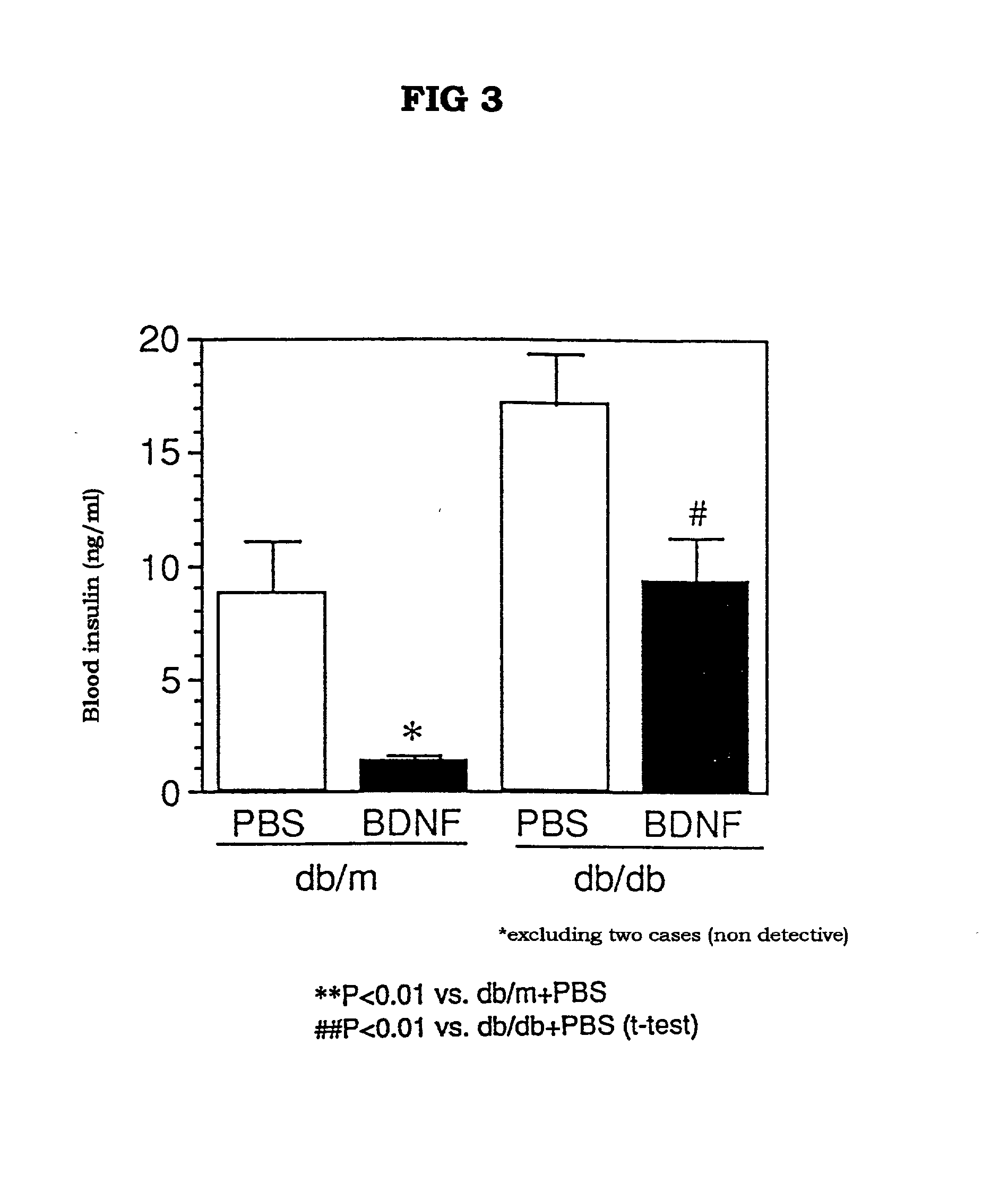
The present invention provides a therapeutic agent for treatment of diabetes and hyperlipemia, especially a therapeutic agent for treatment of type II diabetes mellitus, which comprises as the active ingredient a neurotrophic factor such as BDNF (brain-derived neurotrophic factor), ligands of trkB or trkC receptors, NGF, NT-3, NT-4 / 5, CNTF, GDNF, HGF, etc. Different from conventional oral hypoglycemic agents being mainly used in the treatment of type II diabetes mellitus, the agent of the present invention exhibit blood lipid regulating effects and body fat accumulation regulating effects, in addition to the blood glucose regulating effects. Thus, the agent of the present invention are novel, and can reduce the risk factors in diabetes accompanied by hyperlipemia or obesity, without using any other agent.
Owner:SUMITOMO PHARMA CO LTD
Diagnostic agent for ischemic heart disease risk group
InactiveUS20120122777A1Lower Level RequirementsSuppression problemBiocidePeptide/protein ingredientsDiagnostic agentAdditive ingredient
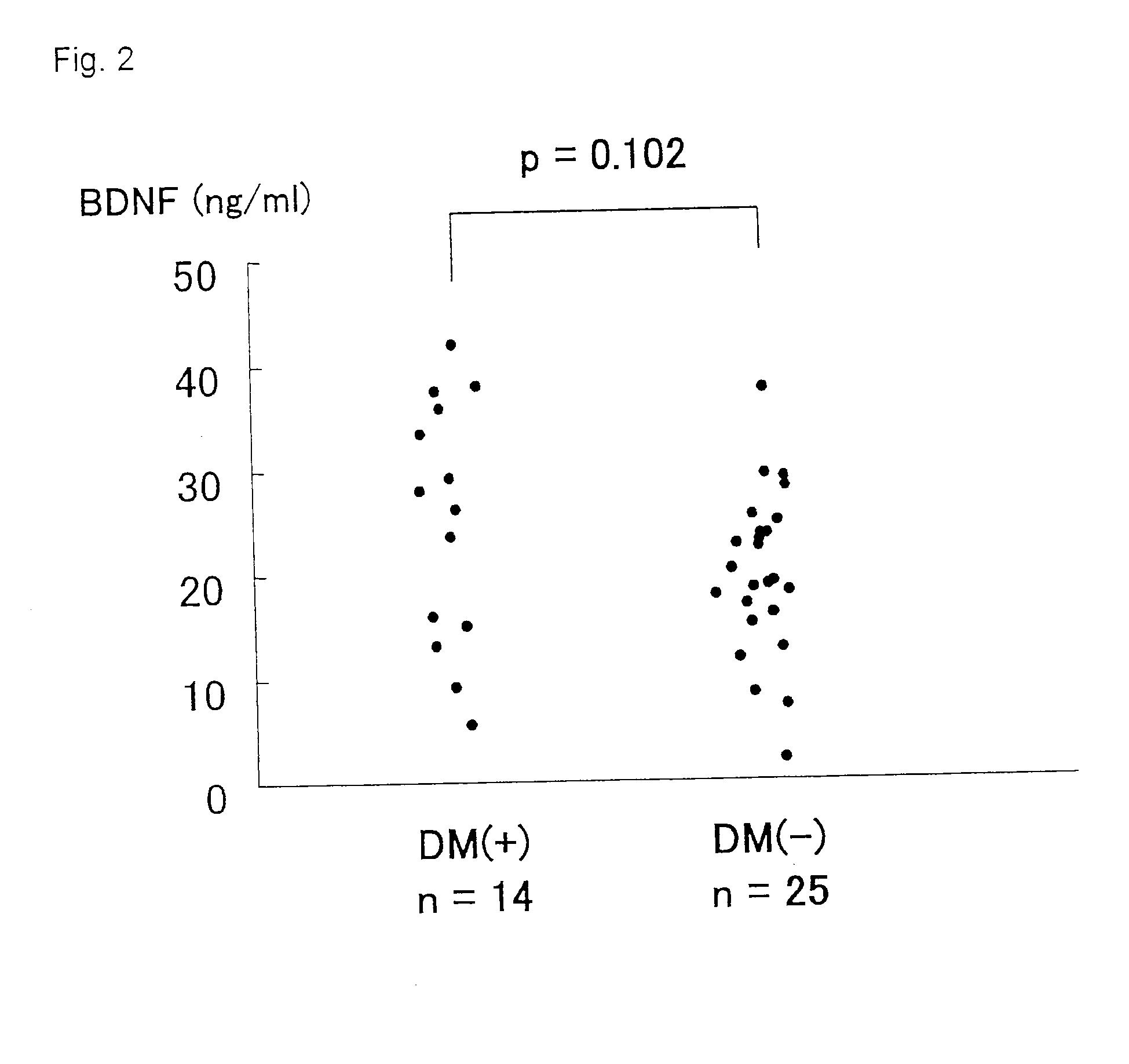
The present invention relates to a diagnostic agent for an ischemic heart disease risk group comprising an anti-brain-derived neurotrophic factor antibody as an effective ingredient, to an assay method for an ischemic heart disease risk group performed by measuring a brain-derived neurotrophic factor concentration in blood, and to a suppressive / preventive drug for ischemic heart disease, particularly for post-infarction myocardial remodeling, comprising a brain-derived neurotrophic factor.
Owner:MASAO DAIMON +1
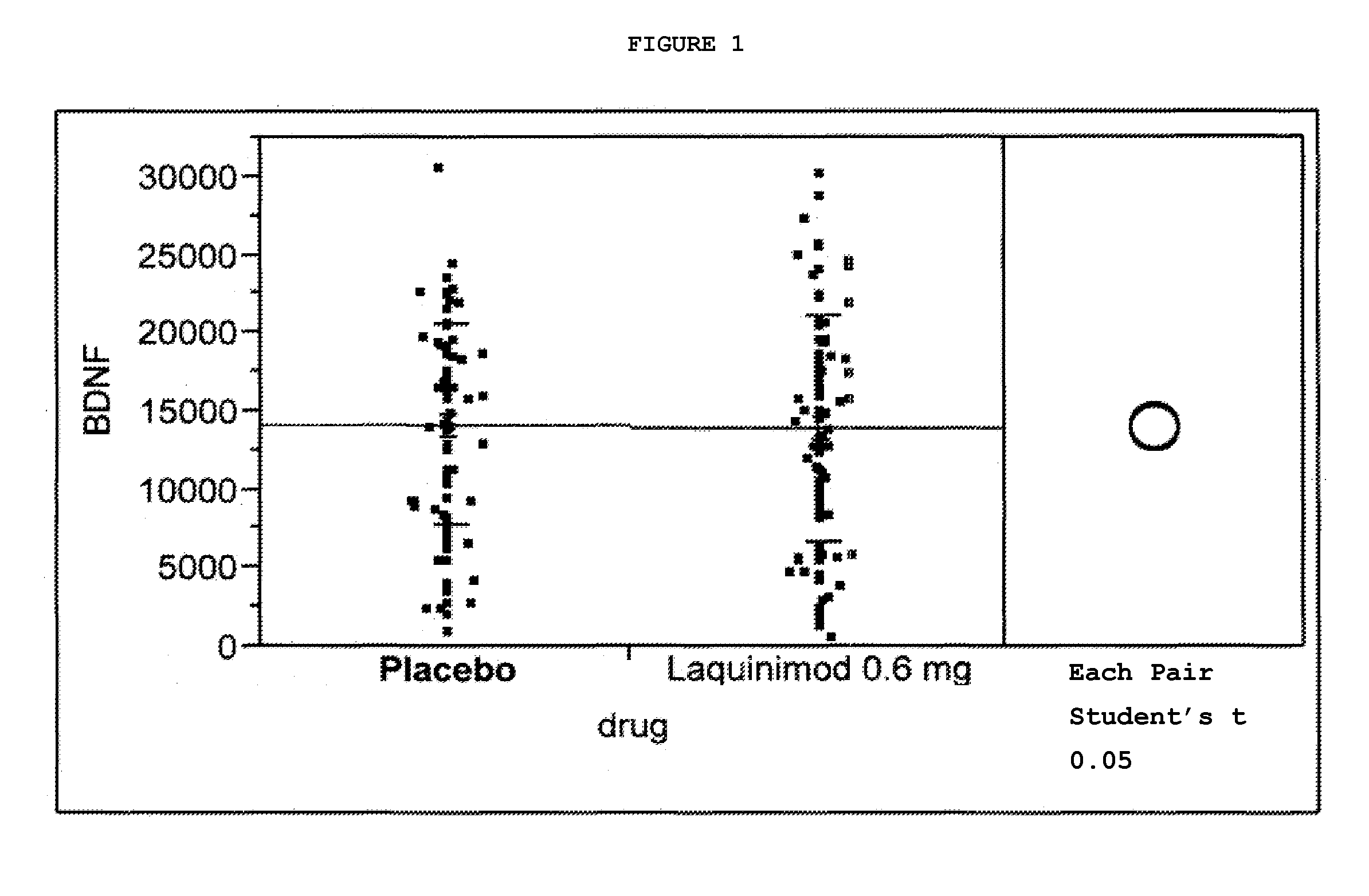
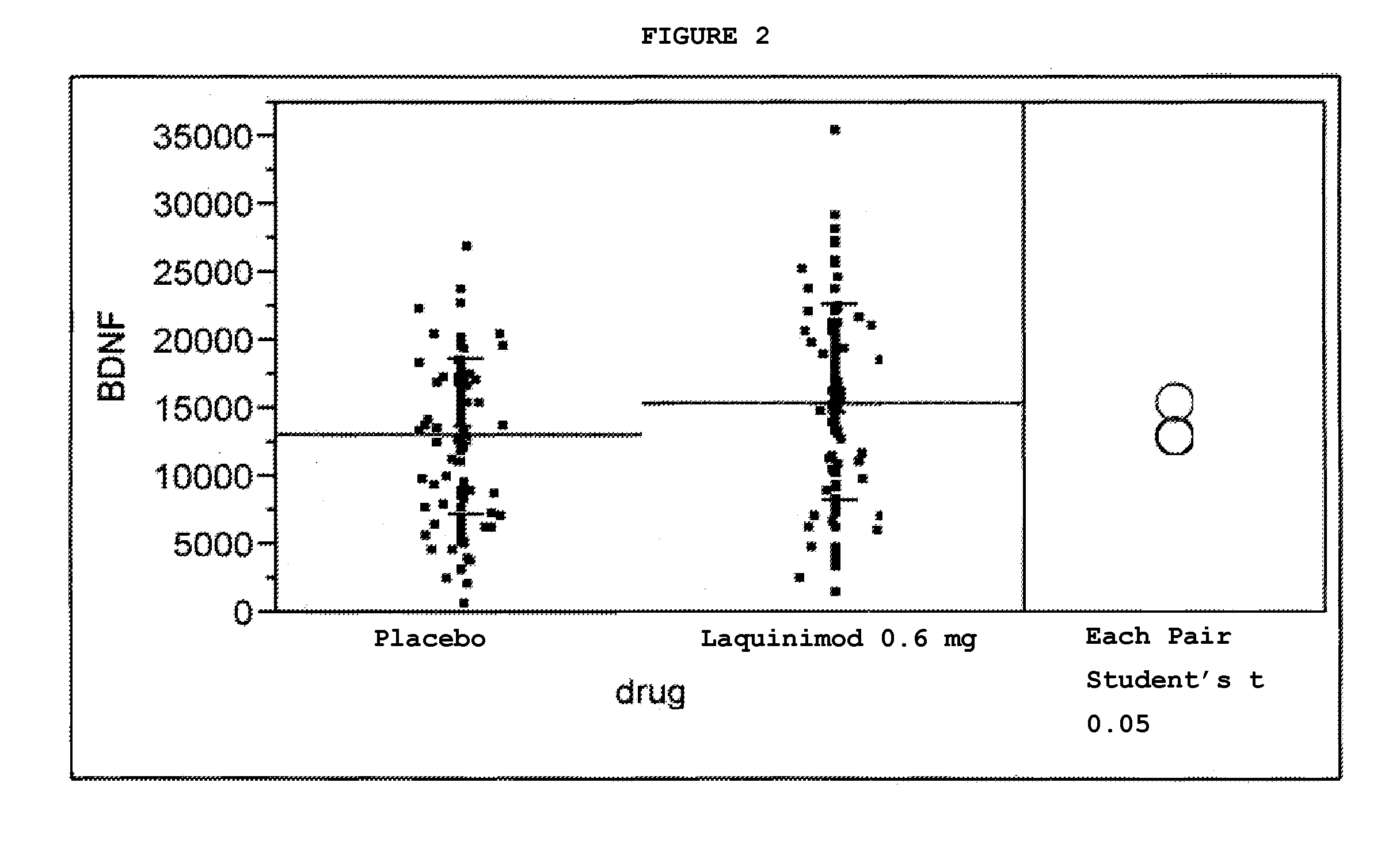
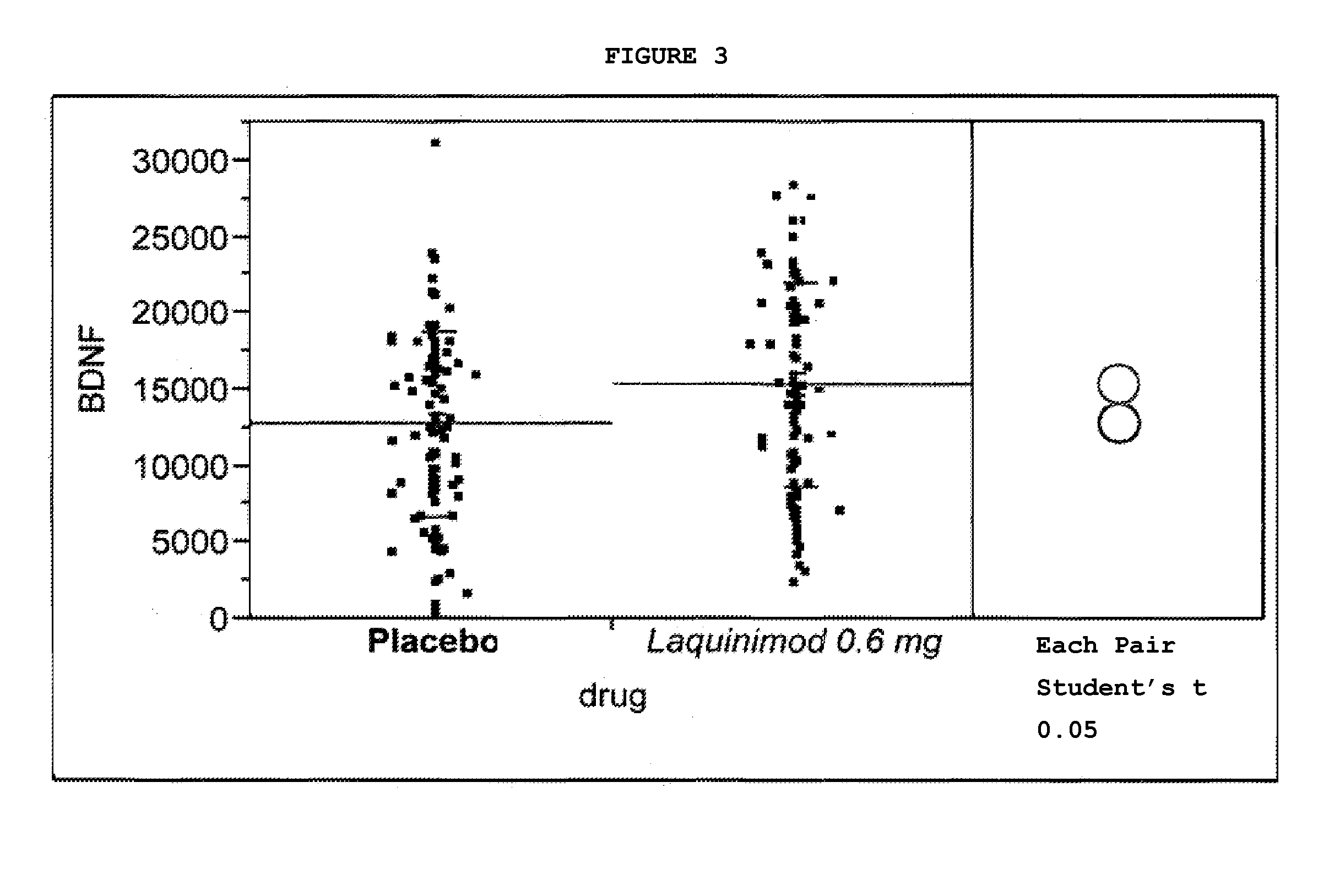
Treatment of BDNF-related disorders using laquinimod
This application provides for a method of increasing brain-derived neurotrophic factor (BDNF) serum level in a human subject comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof effective to increase BDNF serum level in the human subject. The method can further comprise periodically administering to the subject an amount of a second BDNF-increasing agent. This application also provides for a method for treating a human subject suffering from a BDNF-related disease comprising periodically administering laquinimod or a pharmaceutically acceptable salt thereof in an amount effective to treat the human subject. This application additionally provides for use of laquinimod in the manufacture of a medicament for increasing BDNF serum level in a human subject. This application further provides for a pharmaceutical composition comprising an amount of laquinimod effective for use in increasing BDNF serum level in a human subject. This application also provides for a pharmaceutical preparation comprising an amount of laquinimod and an amount of a second BDNF-increasing agent effective for use in increasing BDNF serum level in a human subject.
Owner:ACTIVE BIOTECH AB
Upregulating bdnf levels to mitigate mental retardation
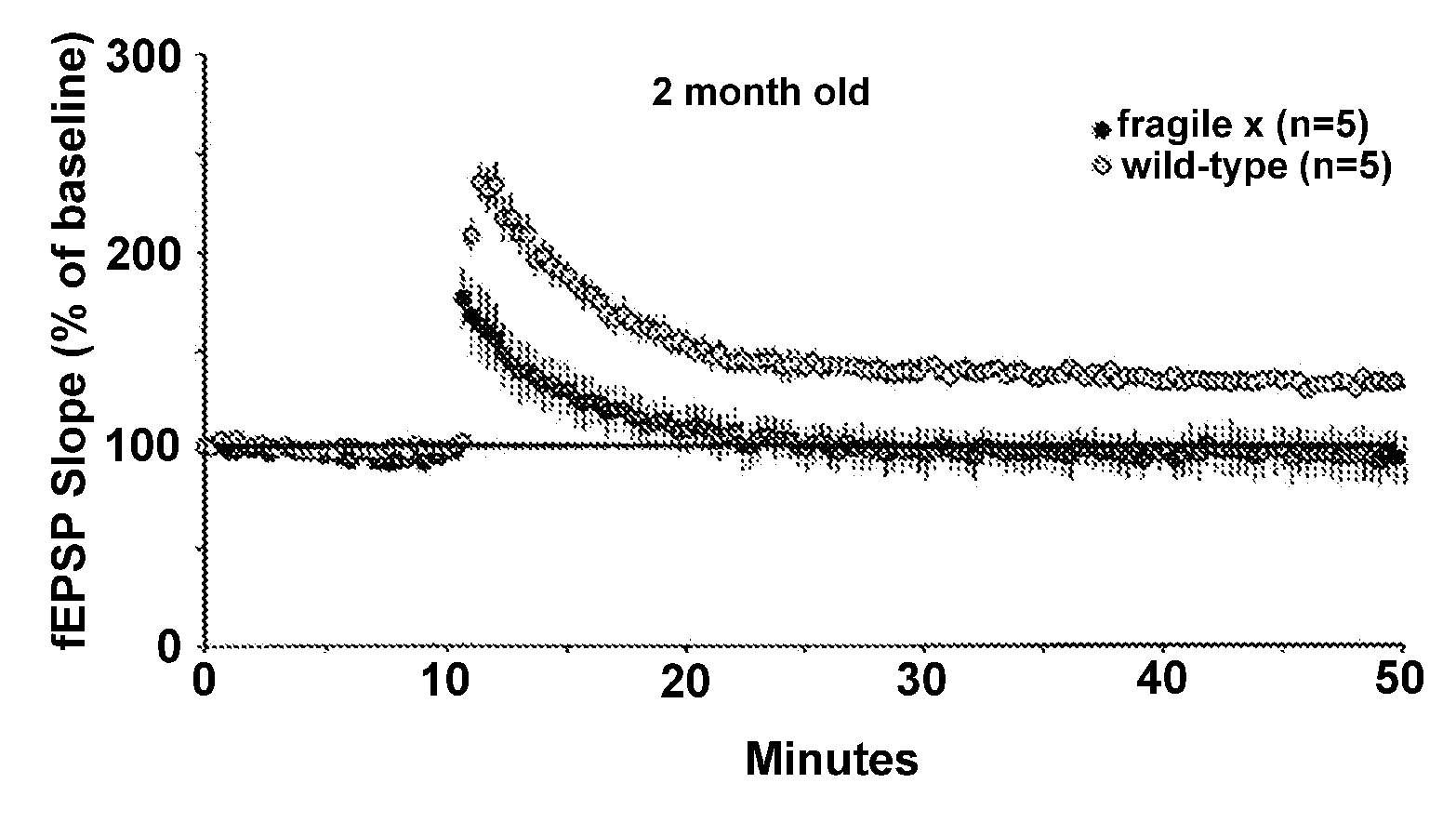
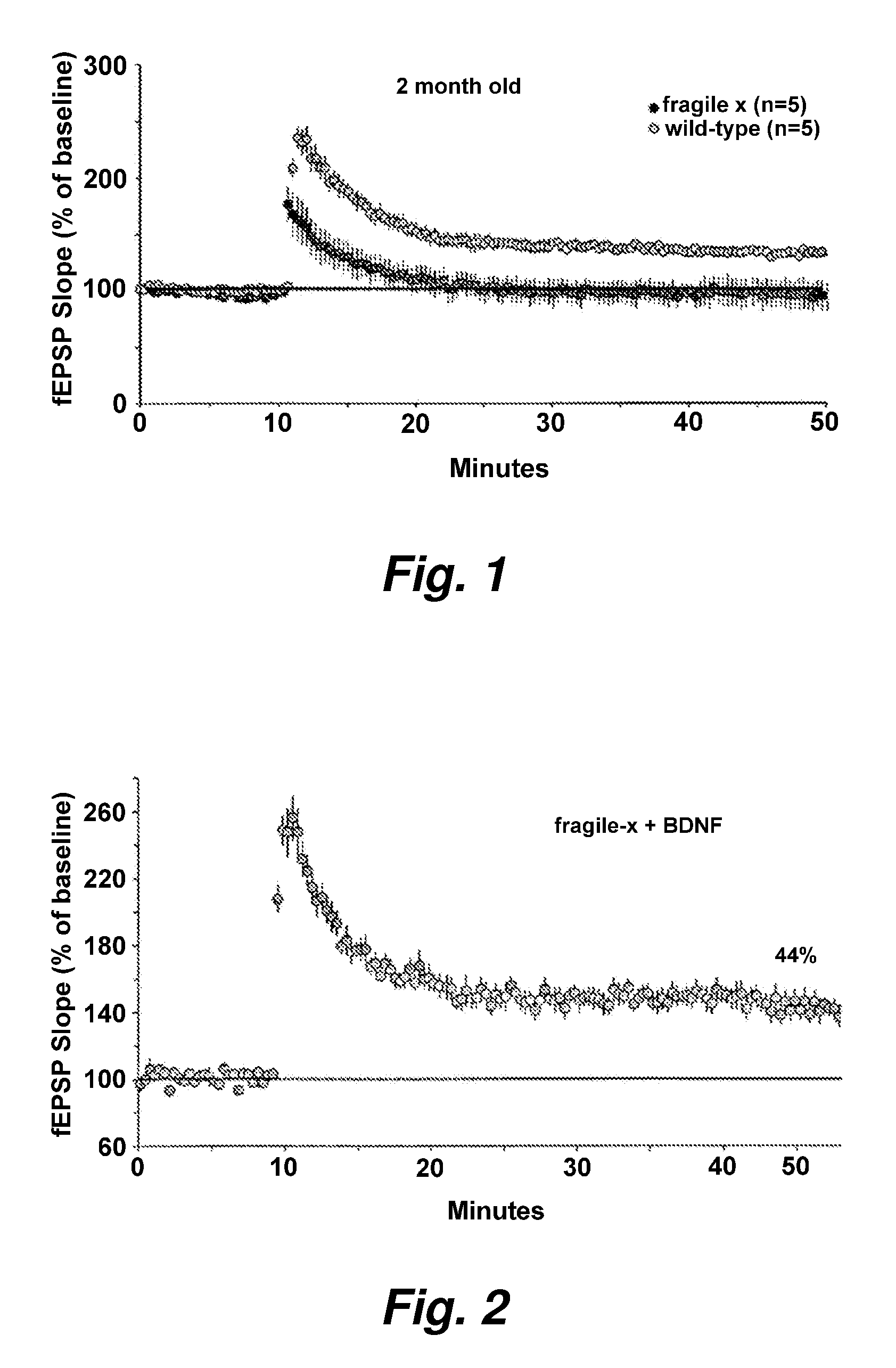
InactiveUS20080139472A1High activityImprove abilitiesBiocideHormone peptidesFragile X chromosomeMammal
This invention provides methods of preserving, improving, or restoring cognitive function in mammal having one or more mutations in the FMR1 gene (e.g. at risk for or having fragile x syndrome), where the methods involve the brain derived neurotrophic factor (BDNF) level or activity in the brain of said mammal. In certain embodiments the methods involve administering one or more AMPA potentiators (e.g., ampakines) to the mammal in an amount sufficient to increase BDNF levels in the brain of the mammal.
Owner:RGT UNIV OF CALIFORNIA
Method(s) of stabilizing and potentiating the actions and administration of brain-derived neurotrophic factor (BDNF)
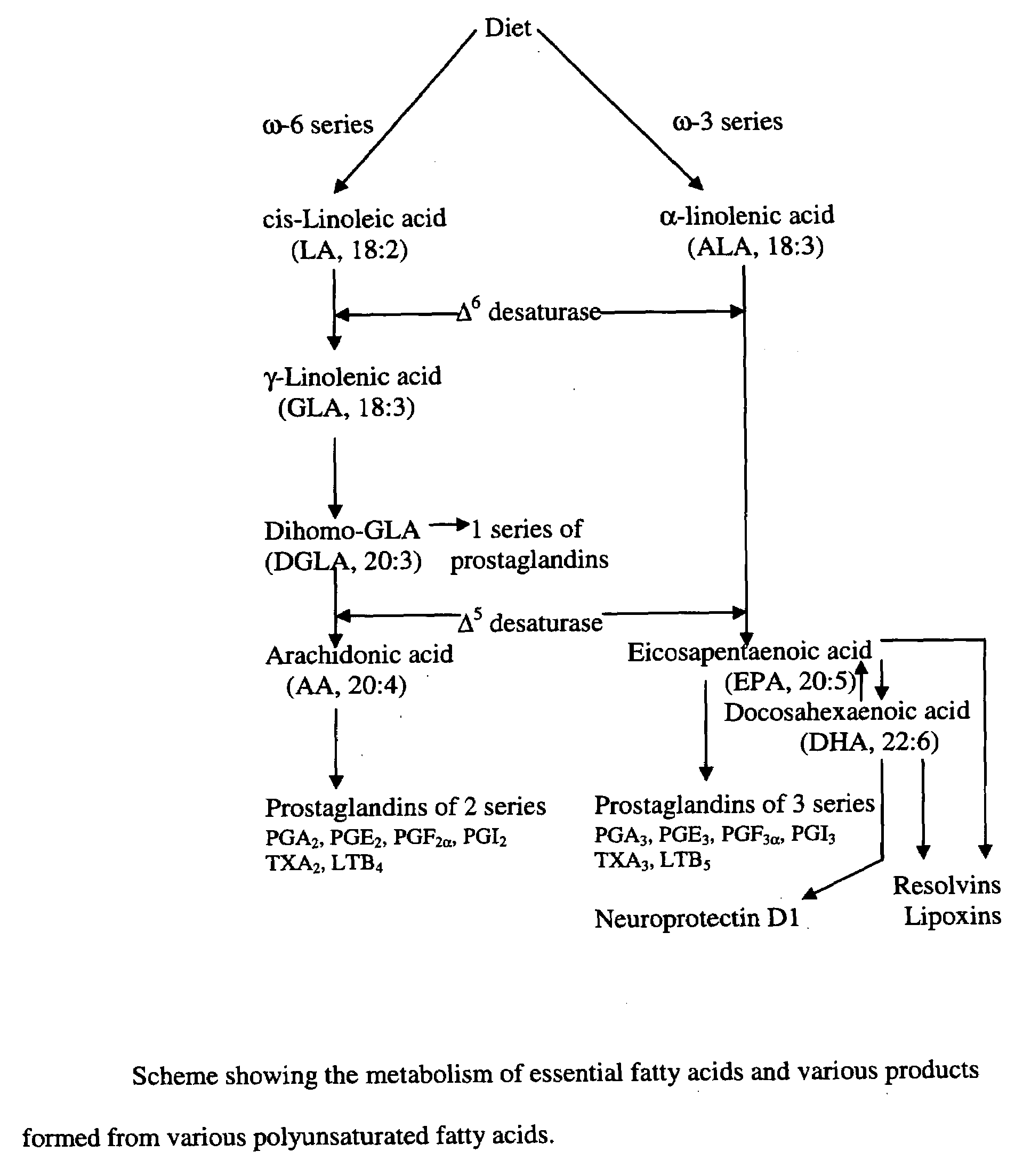
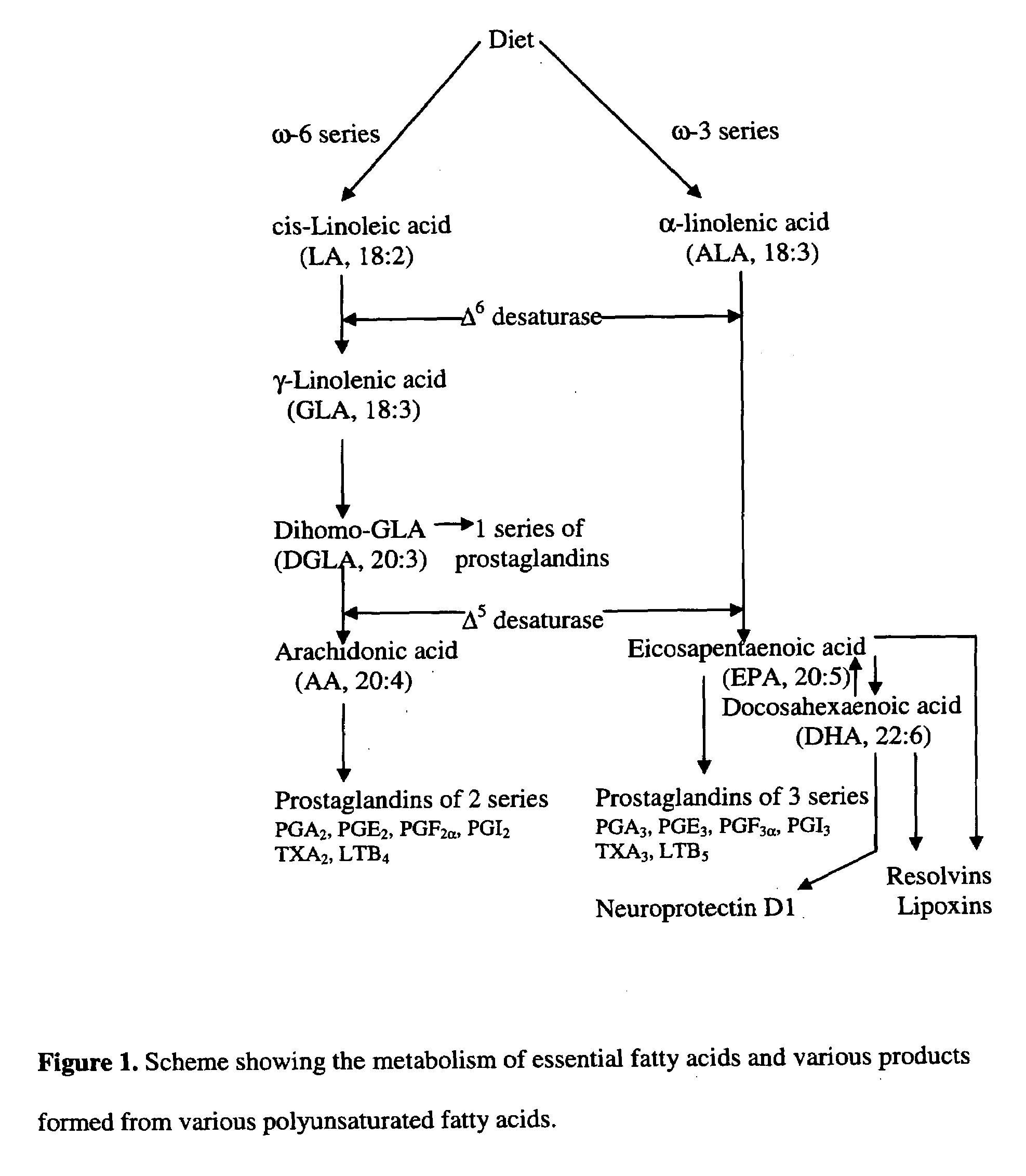
A method of stabilizing and potentiating actions and administration of neurotrophins such as brain-derived neurotrophic factor (BDNF), neurotrophin-3, neurotrophin-4, and nerve growth factor and their receptors by using in coupling conjugation with polyunsaturated fatty acids (PUFAs) in the prevention and / or treatment of obesity, type 2 diabetes mellitus, metabolic syndrome X, Alzheimer's disease, depression, Parkinson's disease, and schizophrenia is described. The invention is directed to the efficacious use of various neurotrophins by using them in combination with polyunsaturated fatty acids chosen from linoleic acid, gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid, alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, cis-parinaric acid, docosapentaenoic acid and conjugated linoleic acid in predetermined quantities. The invention also provides methods of efficiently delivering neurotrophins to the desired areas of the brain by complexing or conjugating with PUFAs, so that they are able to cross blood brain barrier efficiently and reach the desired regions of the brain in adequate amounts.
Owner:DAS UNDURTI N
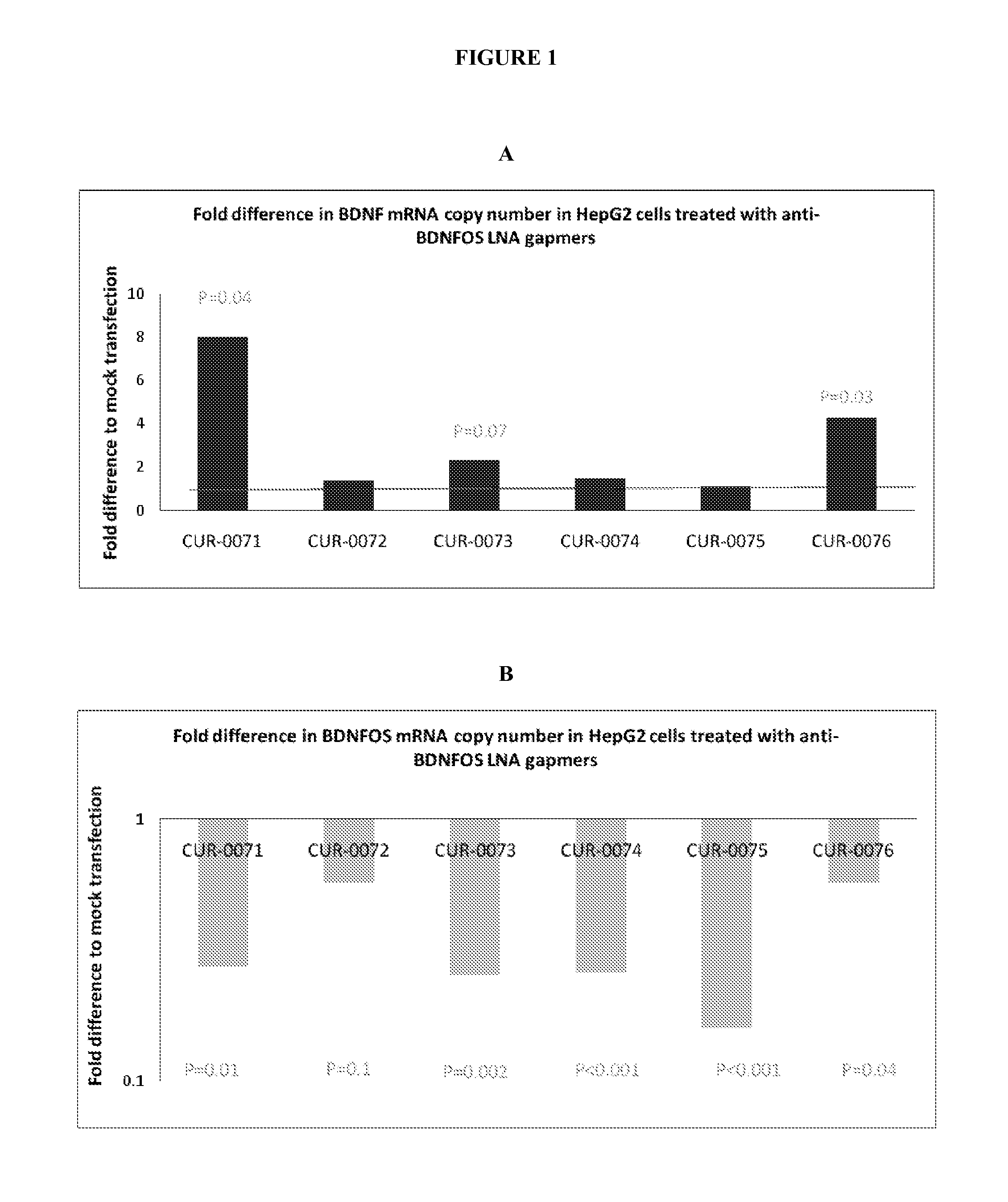
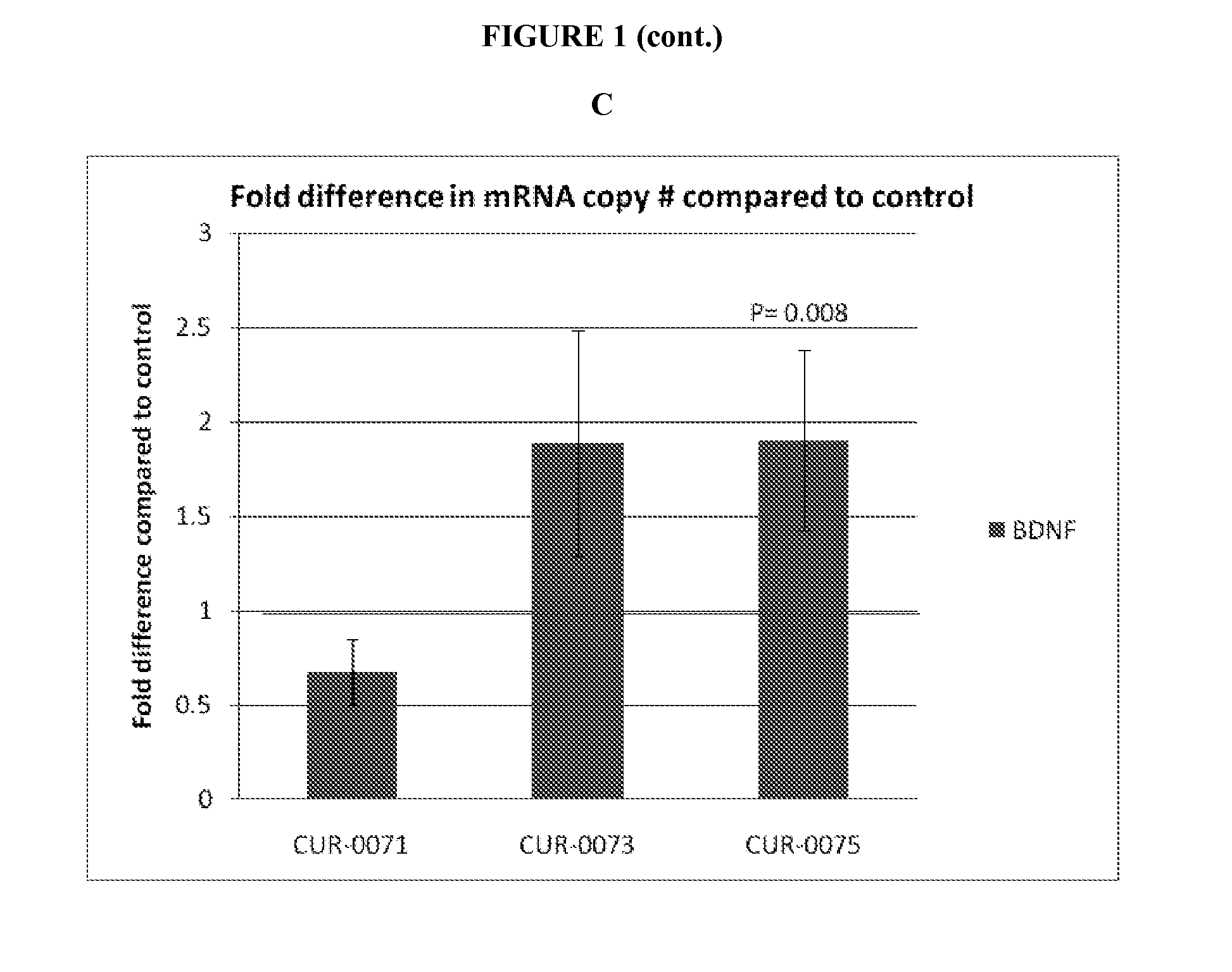
Treatment of brain derived neurotrophic factor (BDNF) related diseases by inhibition of natural antisense transcript to bdnf
ActiveUS20110319475A1Modulate its functionOrganic active ingredientsSenses disorderDiseaseBrain-derived neurotrophic factor
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Brain derived neurotrophic factor (BDNF), in particular, by targeting natural antisense polynucleotides of Brain derived neurotrophic factor (BDNF). The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of BDNF.
Owner:CURNA INC
Probiotic therapies
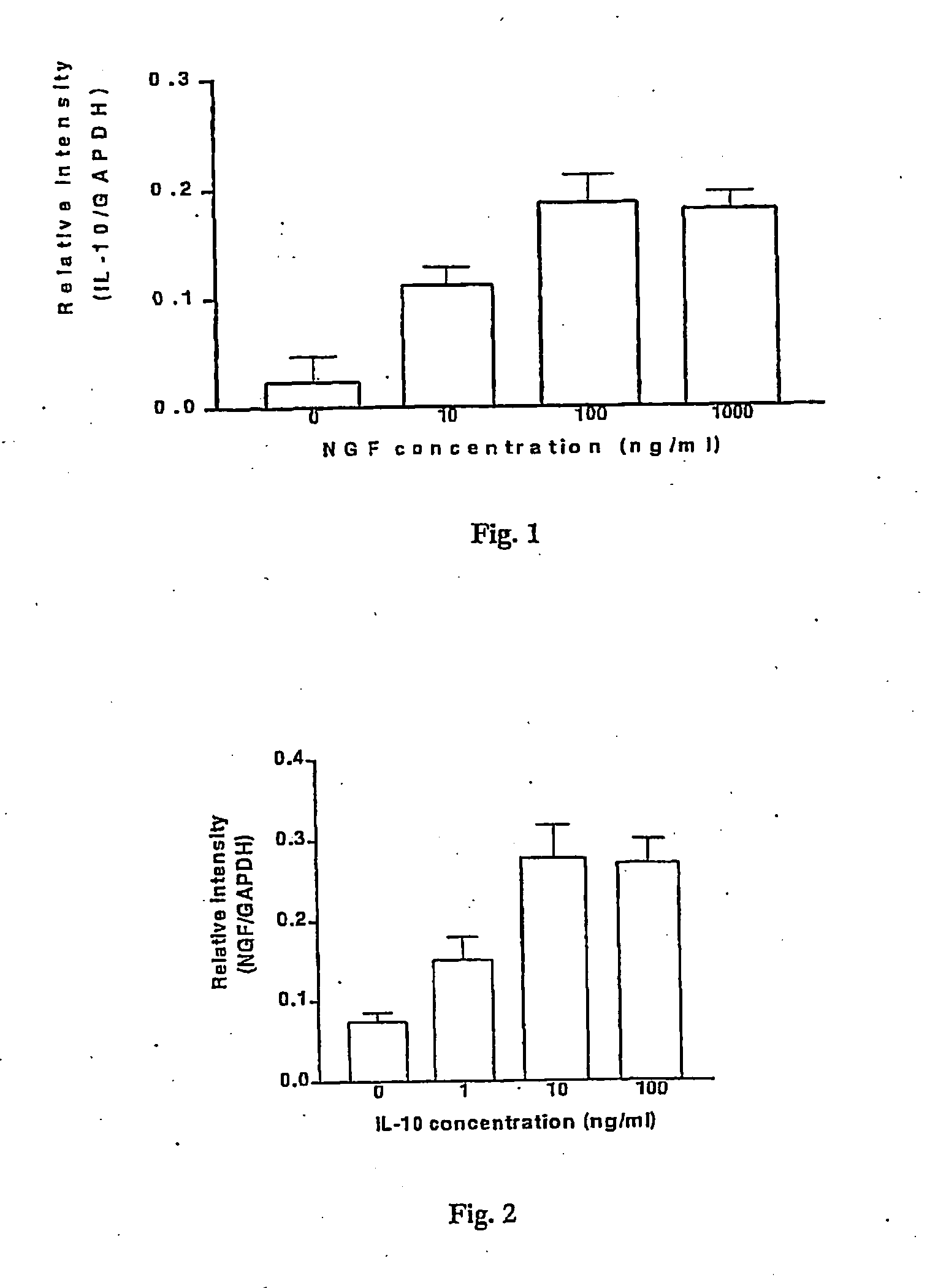
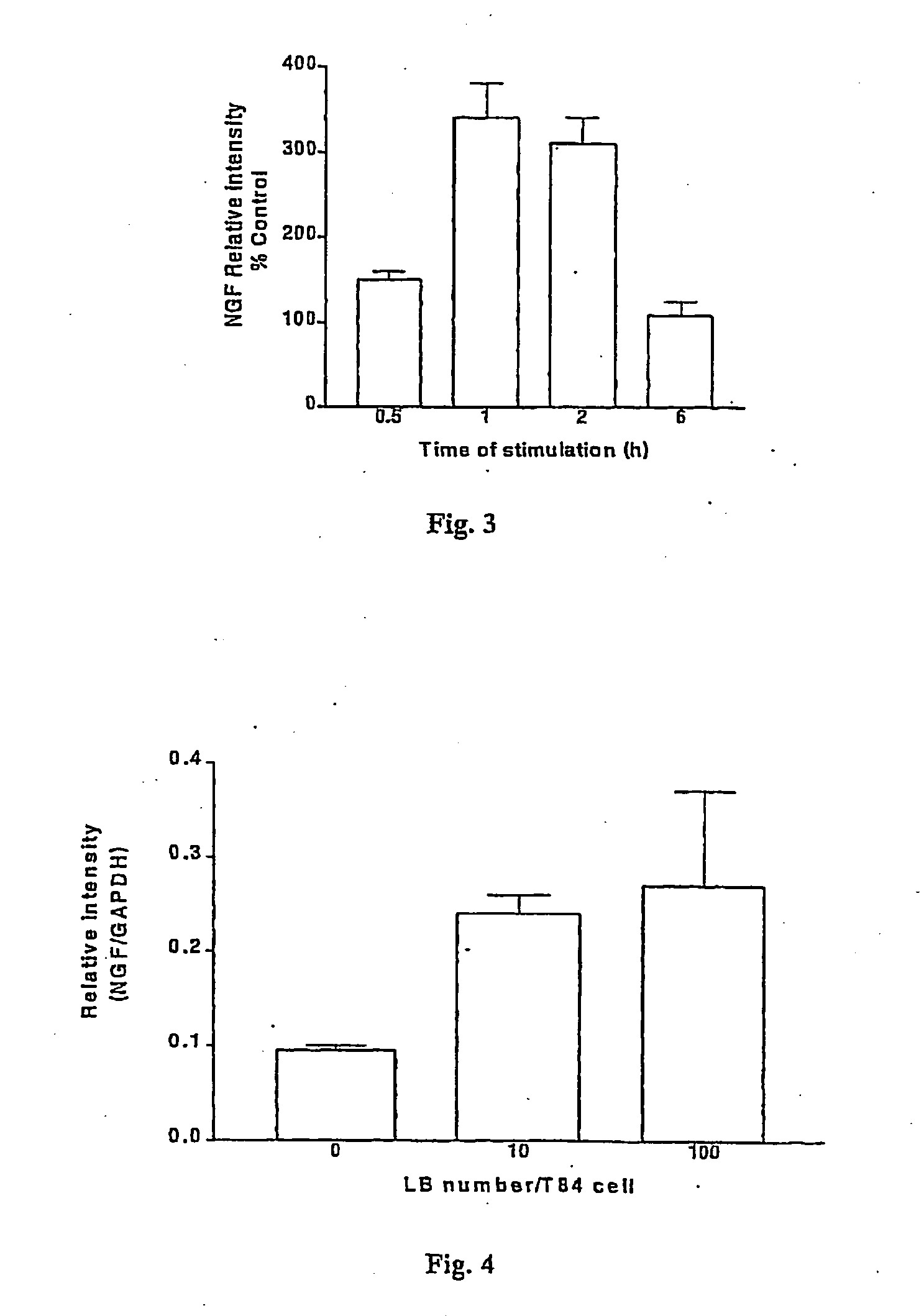
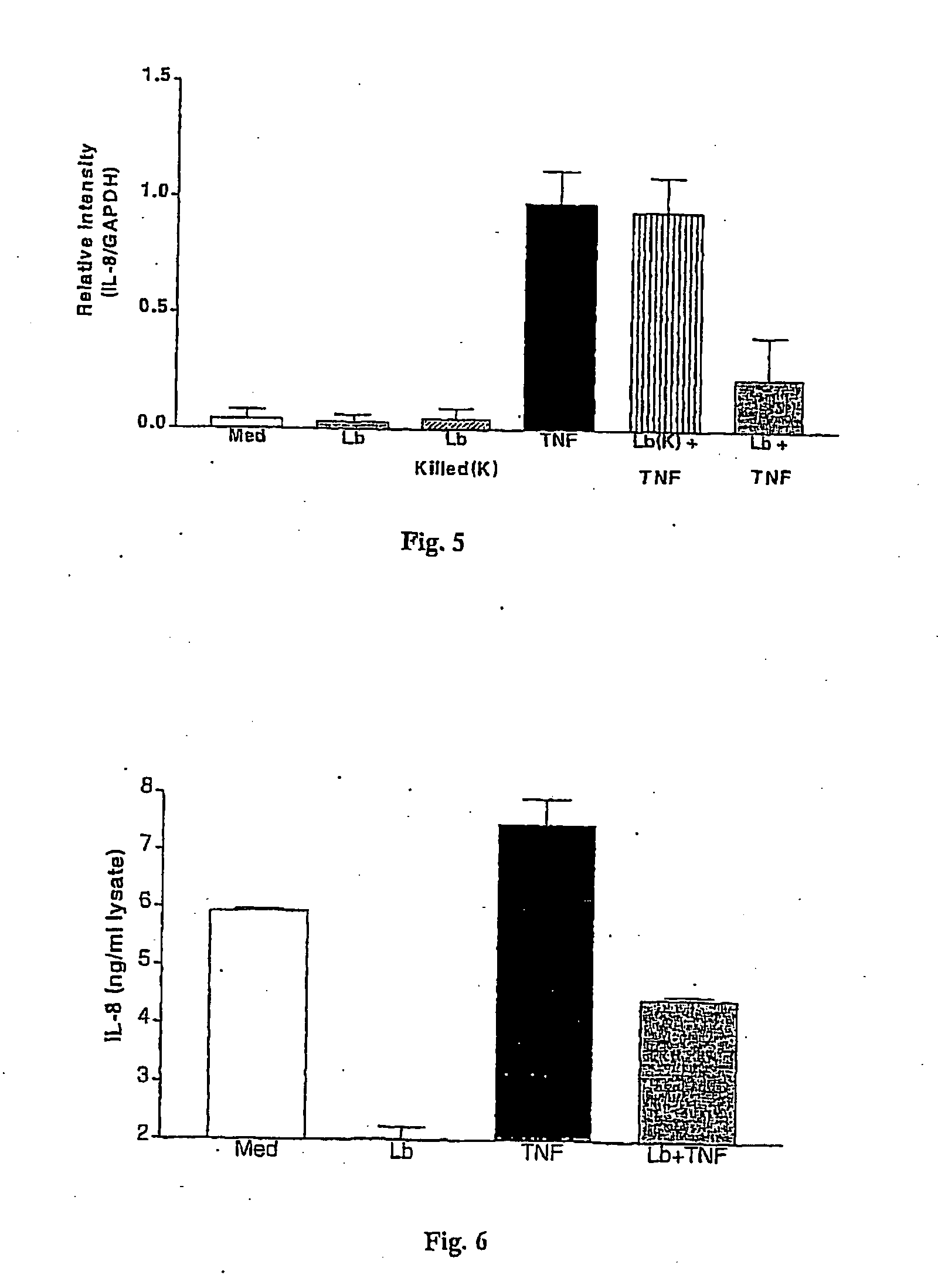
Use of a bacterial strain or an active derivative, fragment or mutant thereof which selectively upregulates the production of nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin 3 (NT3) or neurotrophin 4 (NT4) in the treatment and / or prophylaxis of various disorders especially inflammatory disorders. The bacterial strain may be a Lactobacillus especially,Lactobacillus reuteri.
Owner:UNIV COLLEGE CORK NAT UNIV OF IRELAND CORK
Remedies for diabetes
InactiveUS6391312B1Improve securityBlood glucose level of was not reducedPeptide/protein ingredientsPeptide preparation methodsCvd riskBULK ACTIVE INGREDIENT
The present invention provides a therapeutic agent for treatment of diabetes and hyperlipemia, especially a therapeutic agent for treatment of type II diabetes mellitus, which comprises as the active ingredient a neurotrophic factor such as BDNF (brain-derived neurotrophic factor), ligands of trkB or trkC receptors, NGF, NT-3, NT-4 / 5, CNTF, GDNF, HGF, etc. Different from conventional oral hypoglycemic agents being mainly used in the treatment of type II diabetes mellitus, the agent of the present invention exhibit blood lipid regulating effects and body fat accumulation regulating effects, in addition to the blood glucose regulating effects. Thus, the agent of the present invention are novel, and can reduce the risk factors in diabetes accompanied by hyperlipemia or obesity, without using any other agent.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Recombinant polypeptide useful for neurotrophin receptor mediated gene delivery and as neurotrophin agonist
InactiveUS7205387B2High activityPeptide/protein ingredientsFermentationGene deliveryNeurotrophin Receptor p75
The invention provides a novel recombinant polypeptide that comprises a nucleic acid binding element and a hairpin motif that selectively binds to a neurotrophin receptor. The recombinant polypeptide may be used for neurotrophin receptor mediated delivery of nucleic acid, including therapeutic DNA, bound to the recombinant polypeptide. In one embodiment, the hairpin motif is a hairpin motif of a neurotrophin, such as nerve growth factor, brain derived neurotrophic factor, neurotrophin 3 and neurotrophin 4 / 5. The hairpin motif is also a neurotrophin agonist and therefore may be used to treat any disorder responsive to neurotrophin treatment, such as neurological disorders and tumour. In one embodiment the agonist comprises a hairpin motif that selectively binds to a neurotrophin receptor and a positively charged binding domain which is believed to enhance receptor binding by binding to negatively charged cell membrane.
Owner:AGENCY FOR SCI TECH & RES
Stable pharmaceutical composition of BDNF
InactiveUS6077829AEffective and stableGood storage stabilityPowder deliverySenses disorderActive agentAlcohol sugars
PCT No. PCT / JP97 / 01746 Sec. 371 Date Nov. 16, 1998 Sec. 102(e) Date Nov. 16, 1998 PCT Filed May 26, 1997 PCT Pub. No. WO97 / 45135 PCT Pub. Date Dec. 4, 1997A stable pharmaceutical composition of brain derived neurotrophic factor (BDNF) in the form of an aqueous solution or lyophilized one being suitable for a long-term storage, which contains a surfactant, especially nonionic surfactant (e.g., Tween 80) of 0.001 to 10%, whereby the polymerization and the denaturation of BDNF are inhibited, and the biological activities of BDNF are maintained for a long time. The lyophilized composition can be made more stable by addition of a sugar alcohol (e.g., mannitol) and / or an amino acid (e.g., glycine).
Owner:REGENERON PHARM INC +1
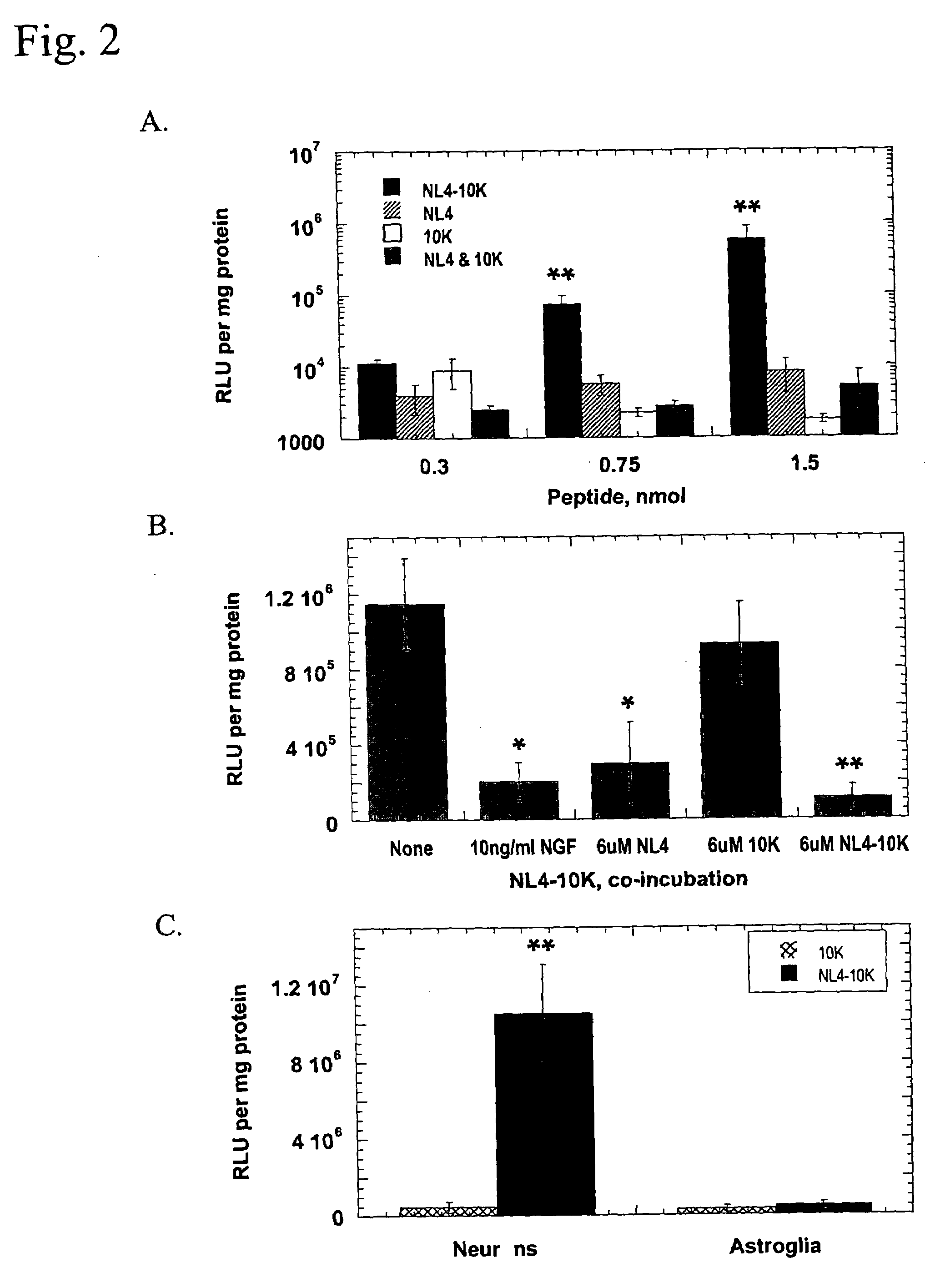
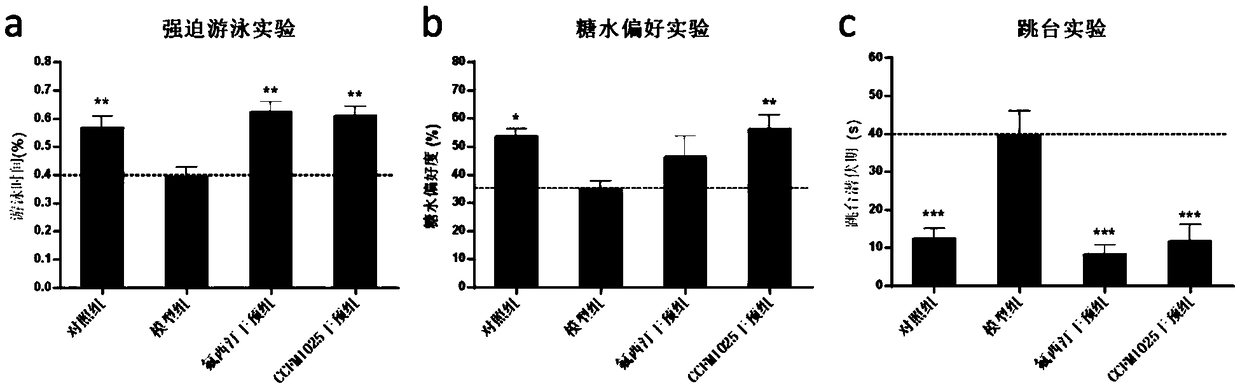
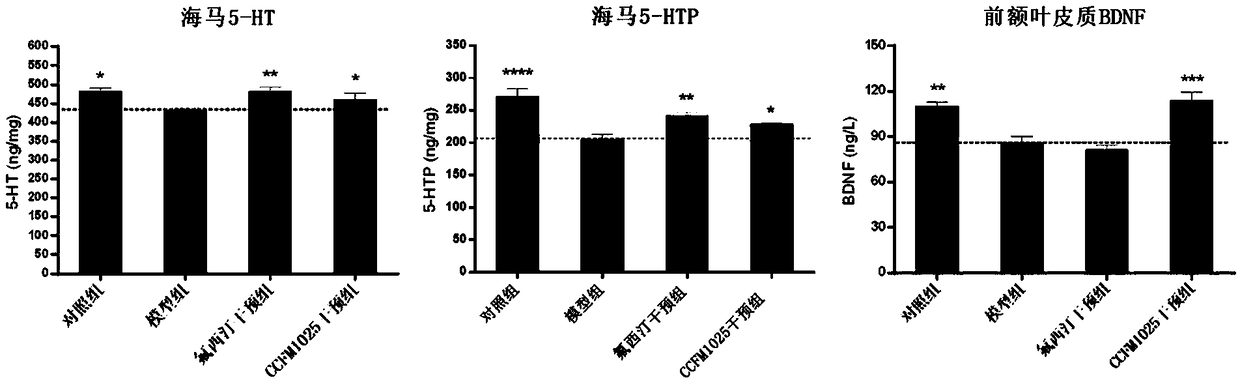
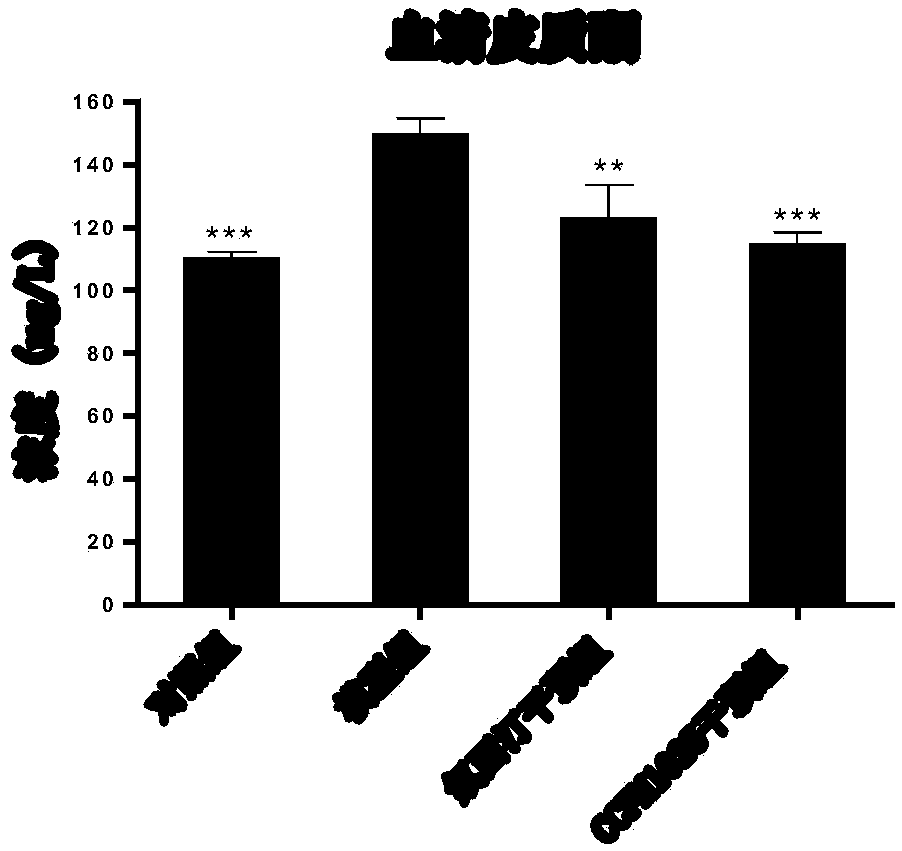
Bifidobacterium breve CCFM1025, fermented food and application thereof
ActiveCN108949640ARelieve depression-like behaviorImprove the level ofNervous disorderBacteriaGut floraEnteropathy
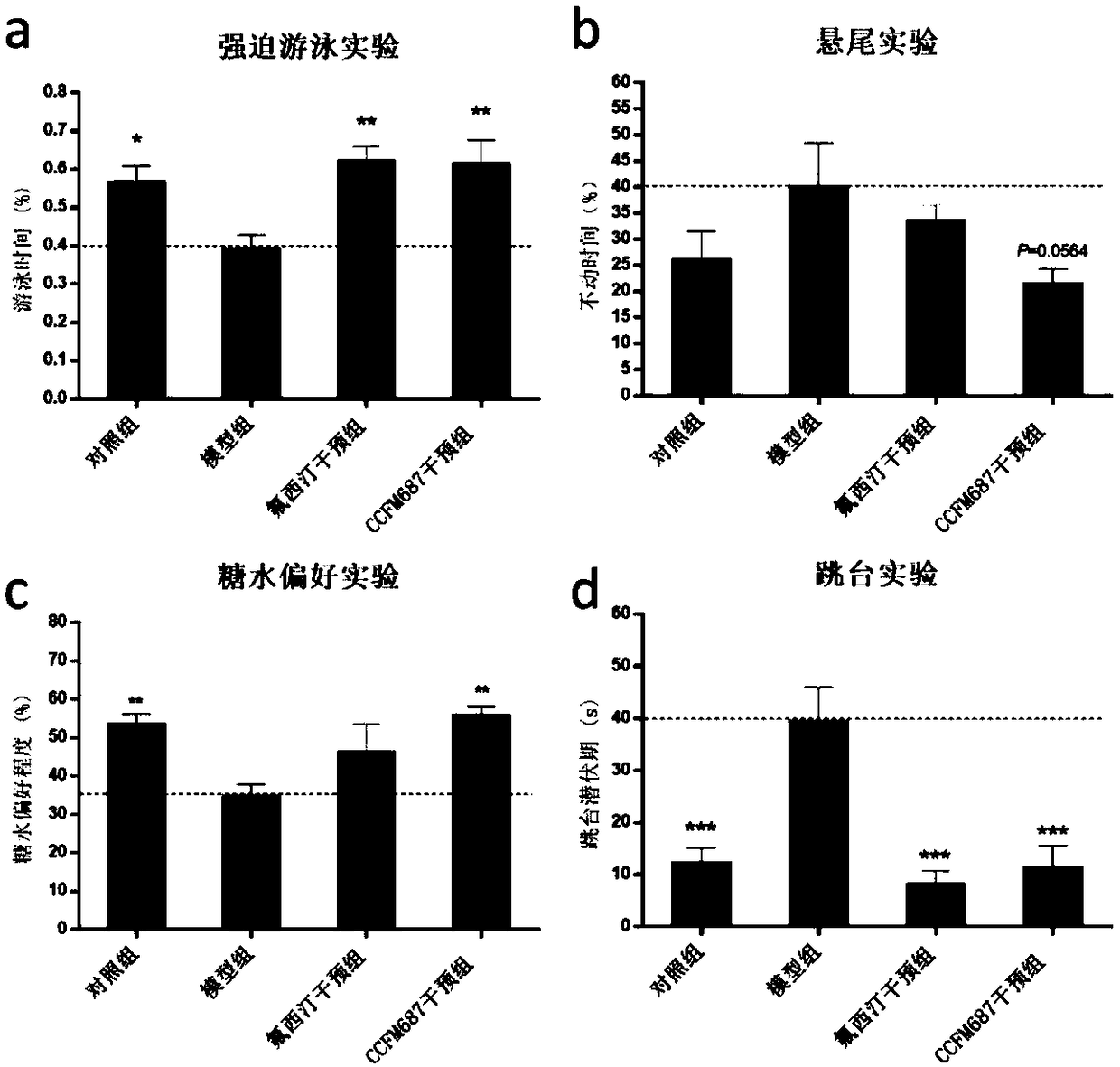
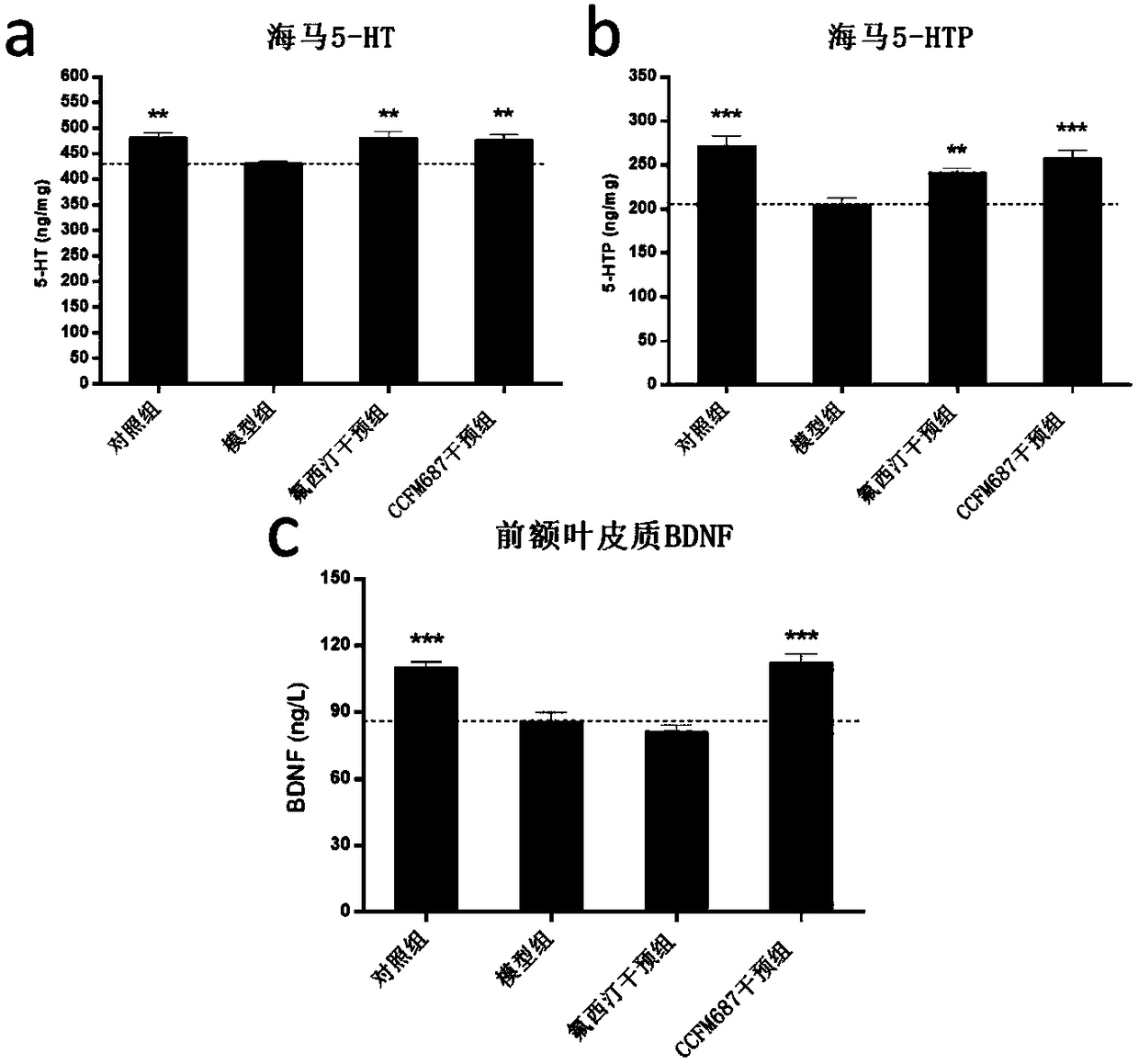
The invention relates to bifidobacterium breve CCFM1025, a fermented food and application thereof. The bifidobacterium breve CCFM1025 can be used for improving the depression-like behavior of a depression mouse, improving 5-hydroxytryptamine in the brain of the depression mouse, improving the level of 5-hydroxytryptamine and a brain-derived neurotrophic factor, reducing the level of corticosteronein serum of the depression mouse, improving the level of 5-hydroxytryptamine in the serum of the depression mouse, improving intestinal flora disturbance of the depression mouse, reducing the abundance of intestinal veillonella, improving the abundance of bifidobacterium and mycoplasmataceae, improving alpha-diversity of intestinal flora and reducing occurrence of inflammatory bowel disease and obesity. By adopting the bifidobacterium breve CCFM1025, the mRNA level of tryptophan hydroxylase in a simulated entero-chromaffin cell can be improved, the secretion volume of 5-hydroxytryptophane ofthe cell can be improved, and a precursor substance is specifically provided to synthesis of 5-hydroxytryptamine in the brain. The bifidobacterium breve CCFM1025 has a wide application prospect.
Owner:无锡食生臻选生物科技有限公司
Recombinant polypeptide useful for neurotrophin receptor mediated gene delivery and as neurotrophin agonist
InactiveUS20050048606A1High resolutionNon invasivePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsGene deliveryNeurotrophin Receptor p75
The invention provides a novel recombinant polypeptide that comprises a nucleic acid binding element and a hairpin motif that selectively binds to a neurotrophin receptor. The recombinant polypeptide may be used for neurotrophin receptor mediated delivery of nucleic acid, including therapeutic DNA, bound to the recombinant polypeptide. In one embodiment, the hairpin motif is a hairpin motif of a neurotrophin, such as nerve growth factor, brain derived neurotrophic factor, neurotrophin 3 and neurotrophin 4 / 5. The hairpin motif is also a neurotrophin agonist and therefore may be used to treat any disorder responsive to neurotrophin treatment, such as neurological disorders and tumour. In one embodiment the agonist comprises a hairpin motif that selectively binds to a neurotrophin receptor and a positively charged binding domain which is believed to enhance receptor binding by binding to negatively charged cell membrane.
Owner:AGENCY FOR SCI TECH & RES
Methods and compositions for treating male erectile dysfunction
InactiveUS7265091B2Peptide/protein ingredientsGenetic material ingredientsFemale Sexual Arousal DisorderNeurotrophin-3
The invention provides a method for preventing or treating male erectile dysfunction or female sexual arousal disorder by administering an effective amount of one or more factors from a group of factors including vascular endothelial growth factor, brain-derived neurotrophic factor, basic fibroblast growth factor, neurotrophin-3, neurotrophin-4, or angiopoietin-1, wherein the factor is a full length protein or a nucleic acid encoding the factor, or a functional derivative or fragment thereof, or an agent that enhances production and / or male erection or female sexual arousal stimulating function of the factor(s). Combinations, kits, and combinatorial methods are also provided.
Owner:RGT UNIV OF CALIFORNIA
Mitigating symptoms and behaviors of substance abuse by modulating GDNF or BDNF pathway activity
InactiveUS20050203011A1Reduces behavioral effect of ethanolDecrease in BDNF levelHormone peptidesMicrobiological testing/measurementSubstance abuserMammal
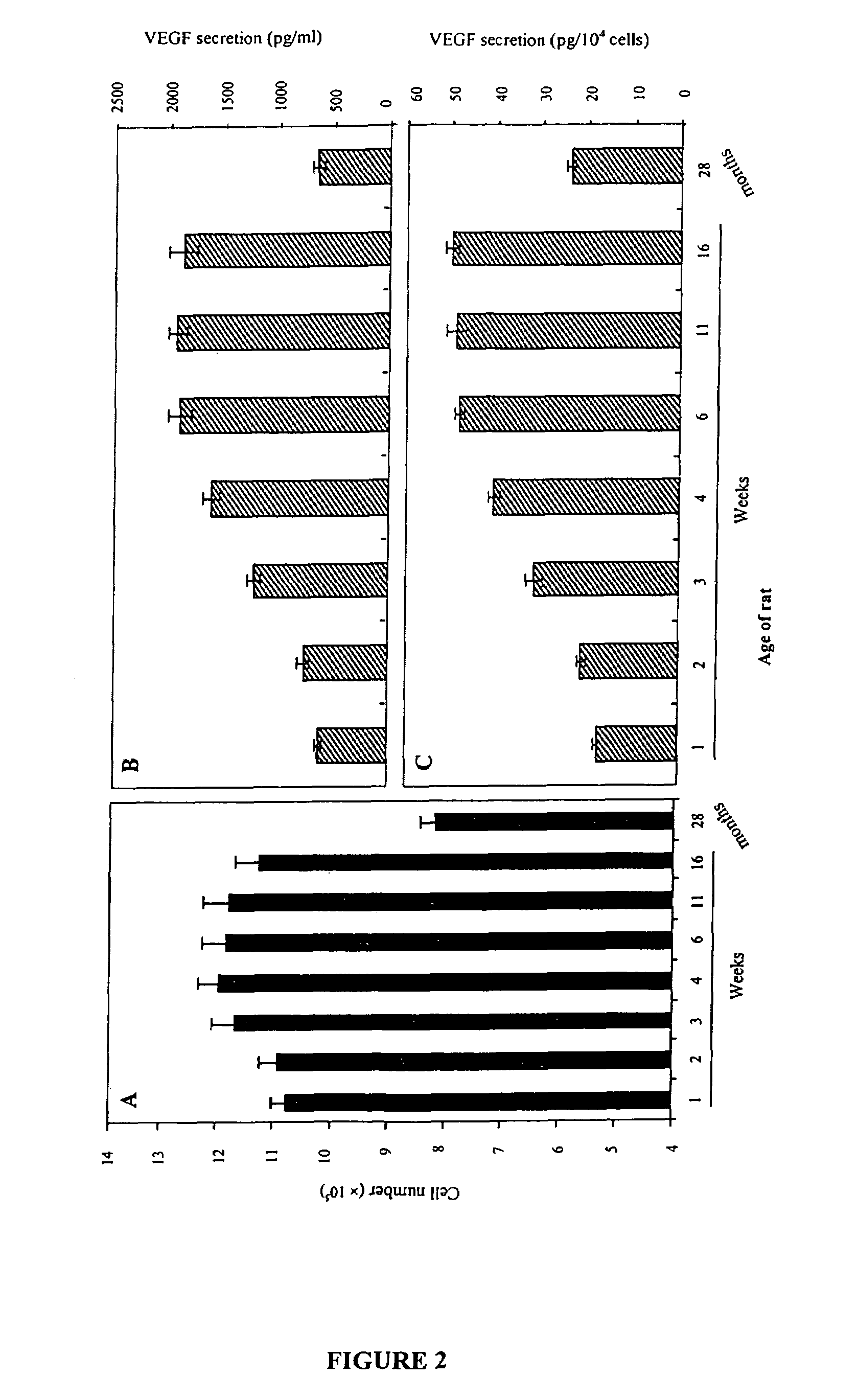
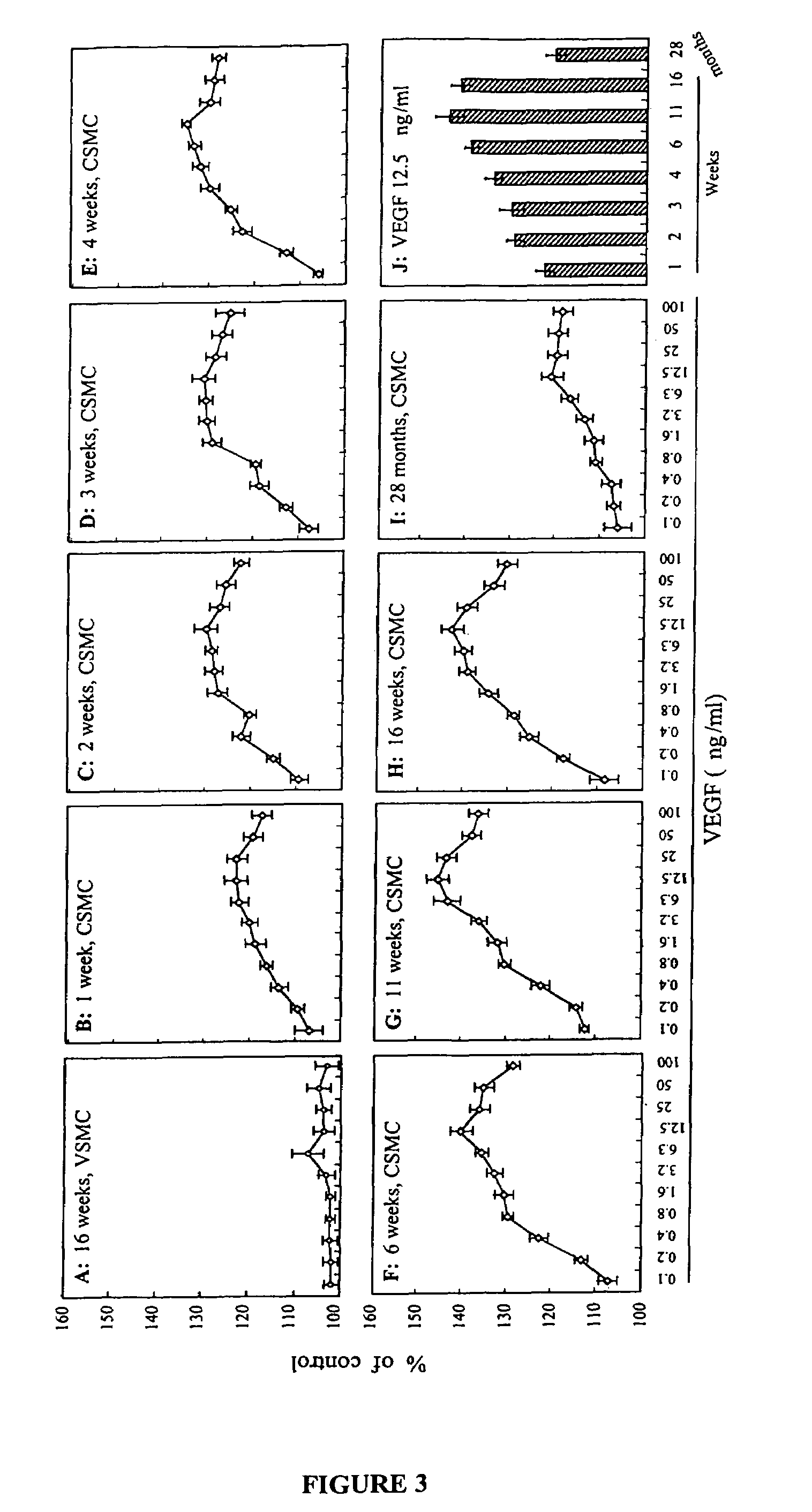
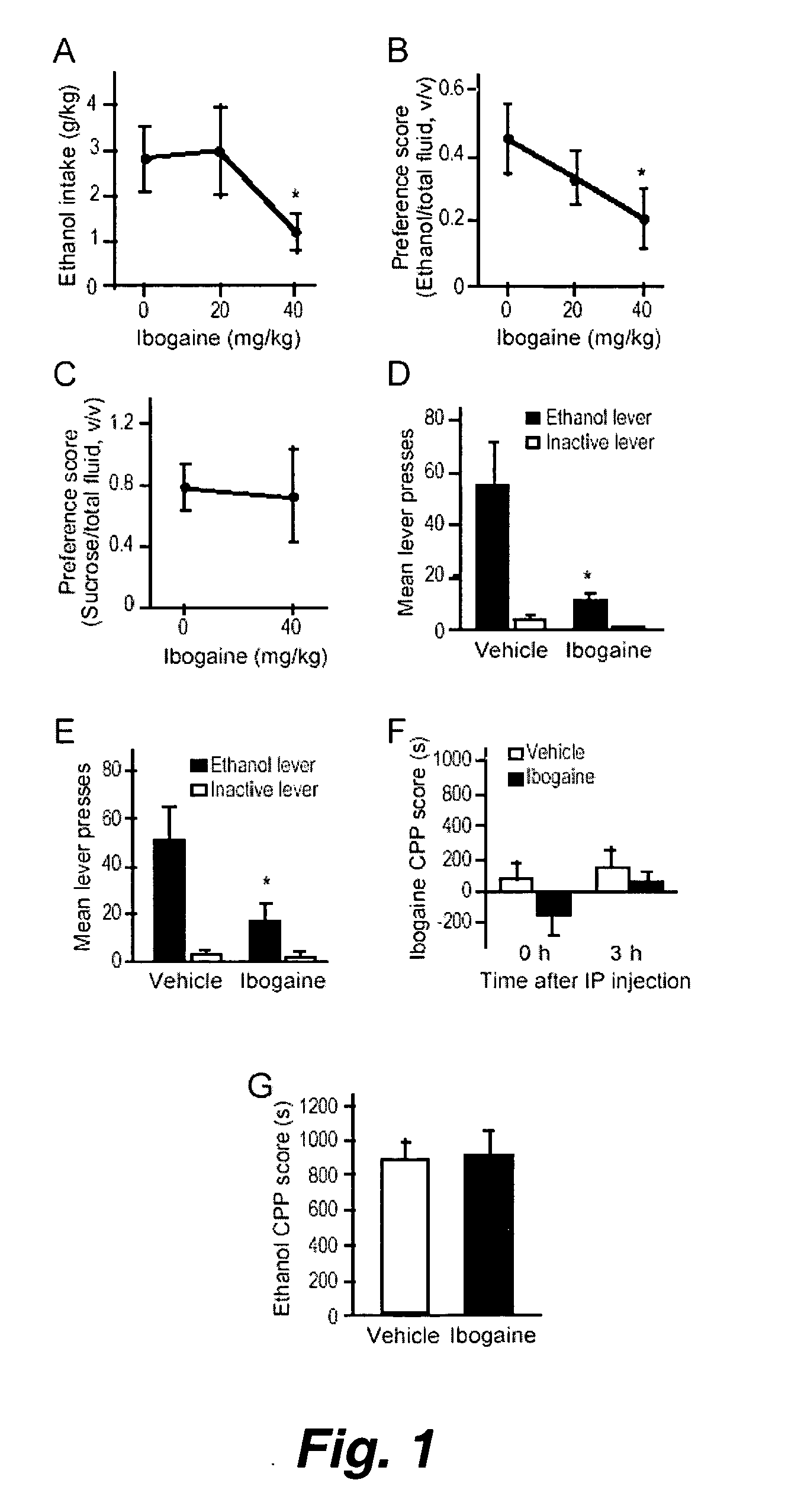
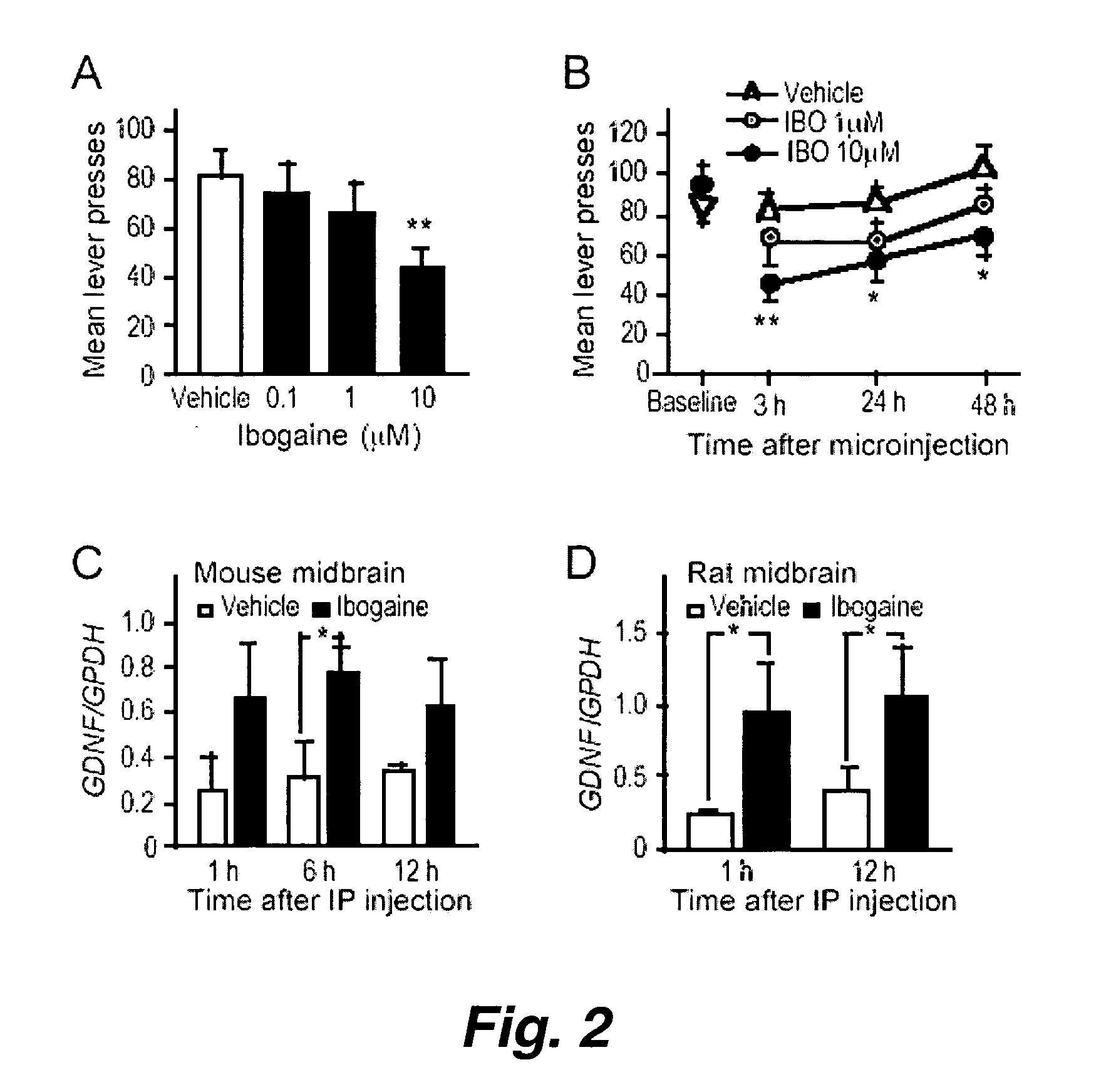
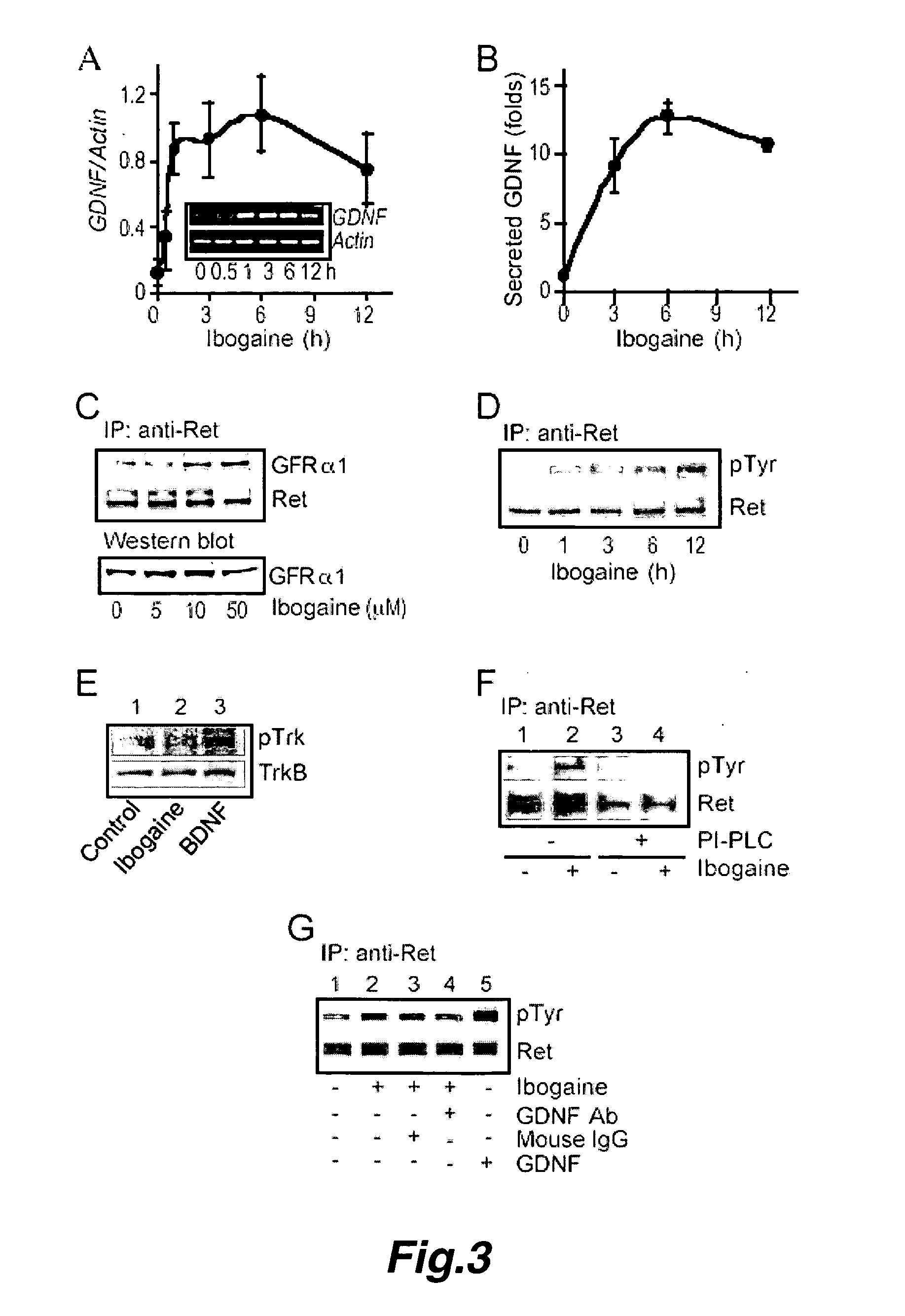
This invention pertains to the discovery that brain-derived neurotrophic factor (BDNF) and its associated signaling pathway is involved in reversing and / or counteracting neuroadaptations within the mesolimbic system that contribute to the development and / or maintenance of addiction (e.g. alcohol addiction). This invention also pertains to the discovery that ibogaine activity is mediated by changes in mRNA expression of GDNF. Thus, in certain embodiments, this inventioni provides a method of mitigating one or more symptoms of substance abuse in a mammal by increasing the expression or activity of GDNF, BDNF, RACK1, and / or the dopamine D3 receptor (D3R) in said mammal.
Owner:RGT UNIV OF CALIFORNIA
Bifidobacterium longum subsp. infantis CCFM687 as well as fermented food and application thereof
ActiveCN109055269ARelieve depression-like behaviorImprove the level ofNervous disorderBacteriaGeneticsGut flora
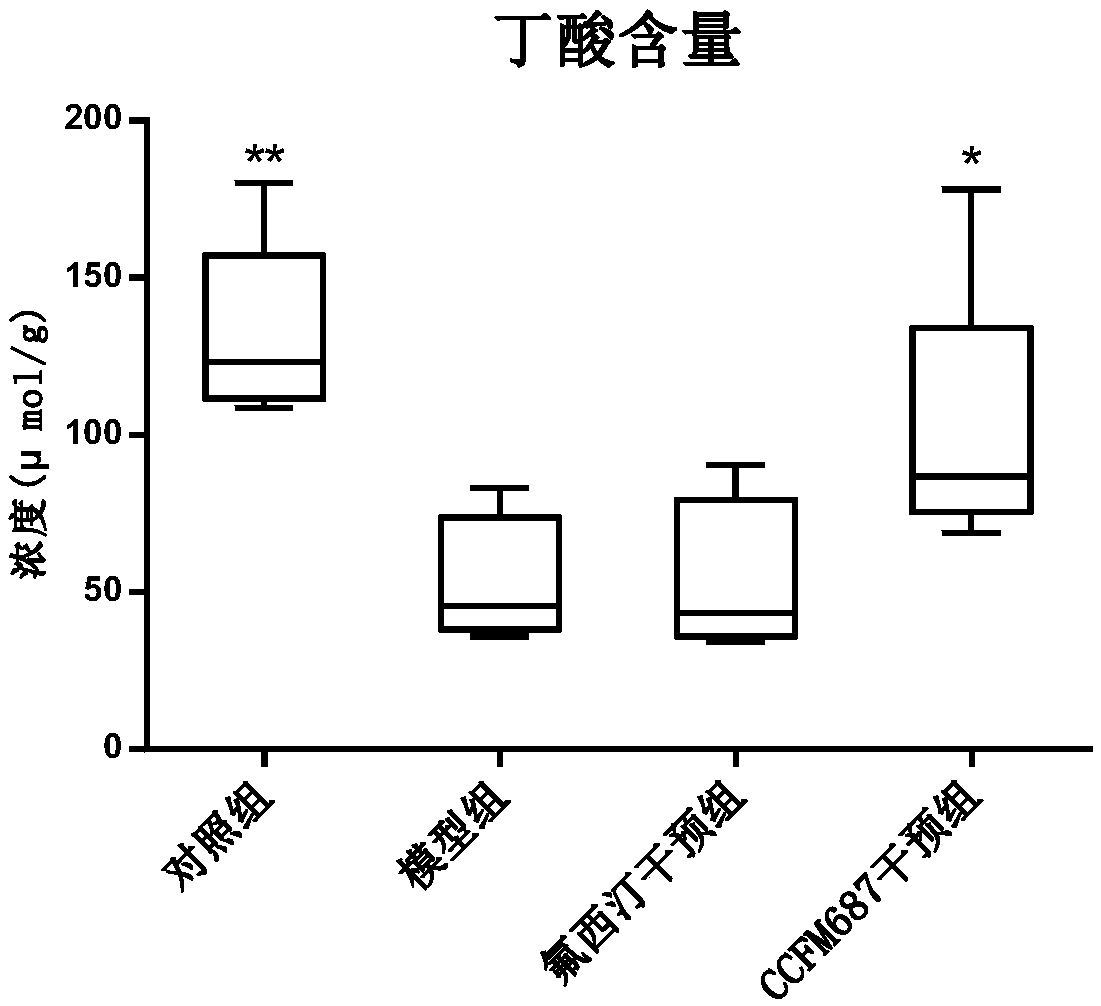
The invention relates to bifidobacterium longum subsp. infantis CCFM687 as well as fermented food and application thereof. The bifidobacterium longum subsp. infantis CCFM687 provided by the inventioncan improve the depressive behavior of a depression mouse and raise the level of 5-hydroxytryptamine, 5-hydroxytryptophane and brain-derived neurotrophic factor in the brain of the depression mouse; moreover, the content of butyric acid in the intestines of the depression mouse is increased; the abundance of intestinal desulfovibrio is lowered while the abundance of bifidobacterium and S24-7 family is raised, the intestinal flora alpha-diversity is improved, the intestinal flora disturbance of the depression mouse is relieved, and the occurrence of autism, inflammatory bowel disease, obesity,diabetes mellitus type I and the like is reduced; the level of mRNA simulating tryptophan hydroxylase in enterochromaffin cell is raised, the secretion of 5-hydroxytryptophane of the enterochromaffincell is increased, and a precursor substance is provided for the synthesis of 5-hydroxytryptamine in brain. Therefore, the bifidobacterium longum subsp. infantis CCFM687 has a broad application value.
Owner:JIANGNAN UNIV
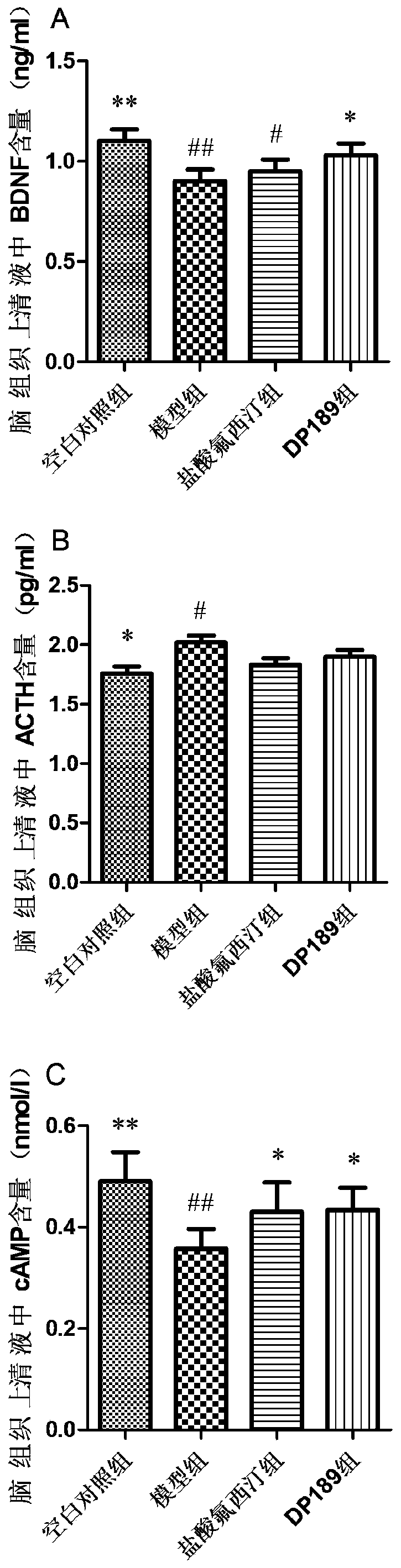
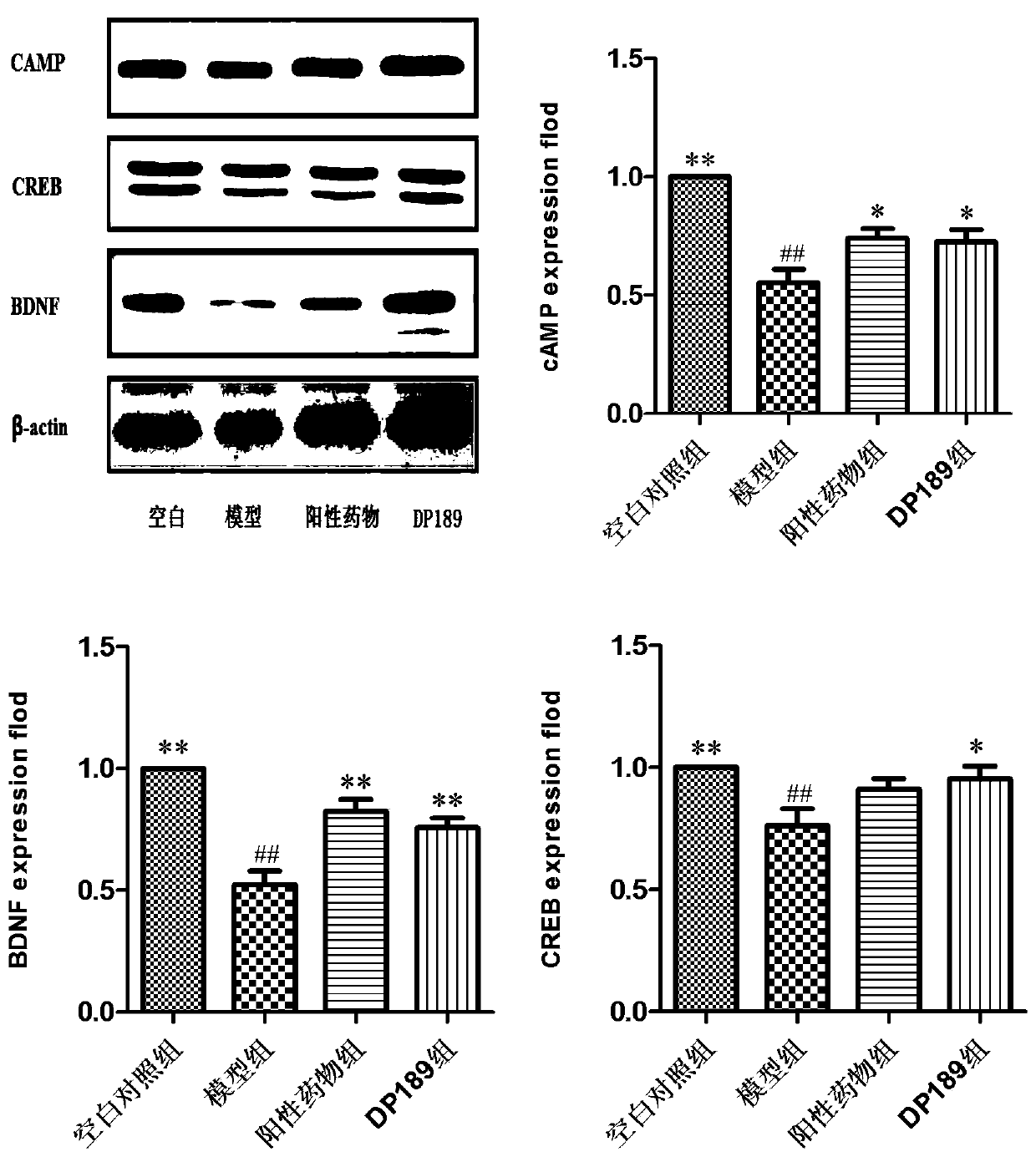
Lactobacillus plantarum DP189 and application thereof
The invention discloses lactobacillus plantarum DP189 and application thereof, and relates to the field of functional food microorganisms. The strain is preserved in China Center for Type Culture Collection on March 25 , 2019 with the preservation number of CCTCC NO:M2019199. By means of the strain, the learning and memory capability of a depressed rat can be improved; the level of brain-derived neurotrophic factors of the depressed rat can be improved; the injury to hippocampal neurons and apoptosis of neuron cells of the depressed rat are reduced; the content of apoptosis proteins JUK2 and Bcl-2 of brain tissue of the depressed rat is lowered; the abundance of verrucomicrobiaceae of the intestinal tract is lowered; the abundance of bacterium lacticum of the intestinal tract is improved, the alpha-diversity of the intestinal floras is improved, and the intestinal flora disturbance of the depressed rat is alleviated. In this way, the lactobacillus plantarum DP189 has good effects of preventing and treating depression / anxiety, infantile autism and inflammatory enteritis. The strain has the anti-depression function, and can serve as a functional probiotic bacterium for preventingand treating the depression, infantile autism and inflammatory enteritis, and a new idea is provided for clinical intervention with and control over depression and other relevant mental diseases.
Owner:JILIN ACAD OF AGRI SCI
Application of phthalide compound
ActiveCN104042606AOrganic active ingredientsNervous disorderTelomeraseBrain-derived neurotrophic factor
An application of a phthalide compound in preparing a medicament. The medicament is specially used for promoting a stem cell to secrete one of the following items: telomerase, a neurotrophic factor (brain-derived neurotrophic factor, BDNF), a stem cell chemotactic factor (stromal cell-derived factor-1, SDF1), a stem cell chemotactic factor receptor (CXC chemokine receptor 4, CXCR4), and an immunoregulatory factor; a kit comprising a phthalide compound and a stem cell is also provided.
Owner:HAWKING BIOLOGICAL TECH
Neurotherapeutic Nanoparticle Compositions and Devices
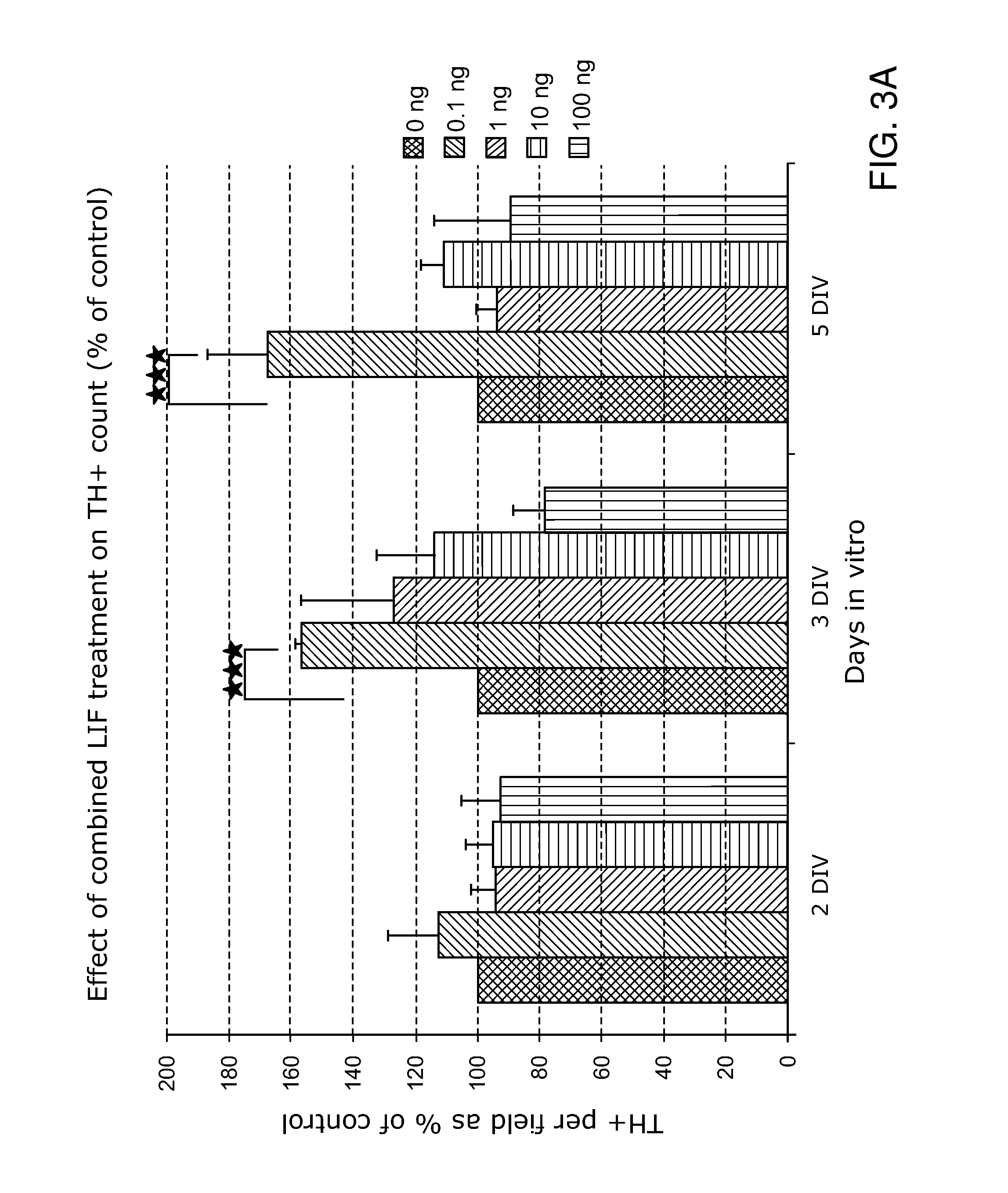
There are provided compositions and methods for treatment of neurodegeneative diseases and CNS injury. The compositions a pharmaceutically acceptable carrier solution; and a plurality of biodegradable nanoparticles, wherein the nanoparticles comprise a targeting moiety that is able to bind selectively to the surface of a neural stem cell and wherein the nanoparticles further comprise factors such as leukaemia inhibitory factor (LIF); XAV939 and / or one or more of : brain-derived neurotrophic factor (BDNF) or an agonist thereof; epidermal growth factor (EGF) or an agonist thereof; glial cell-derived neurotrophic factor (GDNF) or an agonist thereof; retinoic acid and derivatives thereof; ciliary neurotrophic factor (CTNF) or an agonist thereof; and Wnt5A. The biodegradable nanoparticles may deliver via controlled time release.
Owner:YALE UNIV
Therapeutic agent for treatment of diabetes
InactiveUS20020122829A1Lower blood sugar levelsLower Level RequirementsPeptide/protein ingredientsMetabolism disorderTherapeutic effectCvd risk
The present invention provides a therapeutic agent for treatment of diabetes and hyperlipemia, especially a therapeutic agent for treatment of type II diabetes mellitus, which comprises as the active ingredient a neurotrophic factor such as BDNF (brain-derived neurotrophic factor), ligands of trkB or trkC receptors, NGF, NT-3, NT-4 / 5, CNTF, GDNF, HGF, etc. Different from conventional oral hypoglycemic agents being mainly used in the treatment of type II diabetes mellitus, the agent of the present invention exhibit blood lipid regulating effects and body fat accumulation regulating effects, in addition to the blood glucose regulating effects. Thus, the agent of the present invention are novel, and can reduce the risk factors in diabetes accompanied by hyperlipemia or obesity, without using any other agent.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
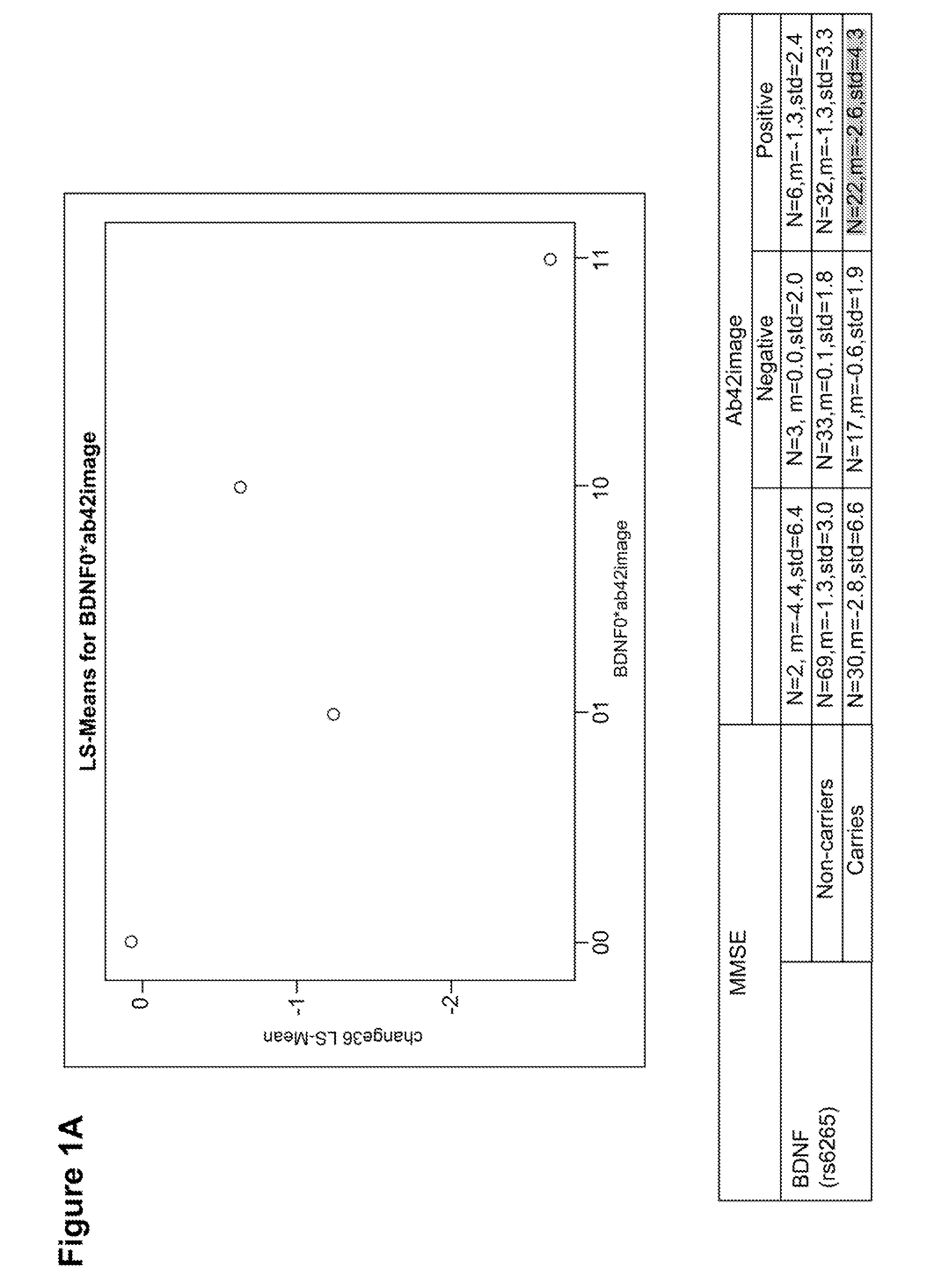
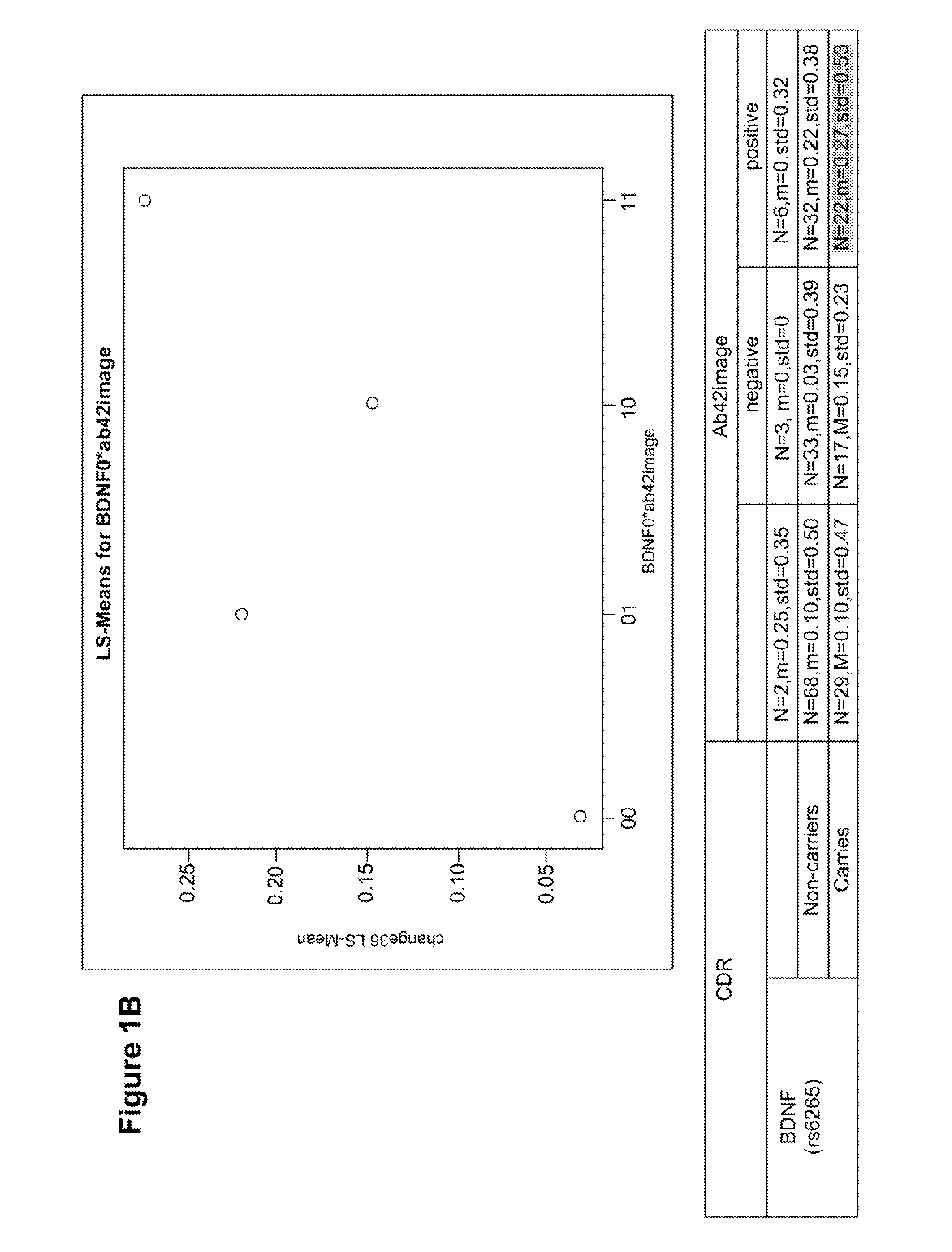
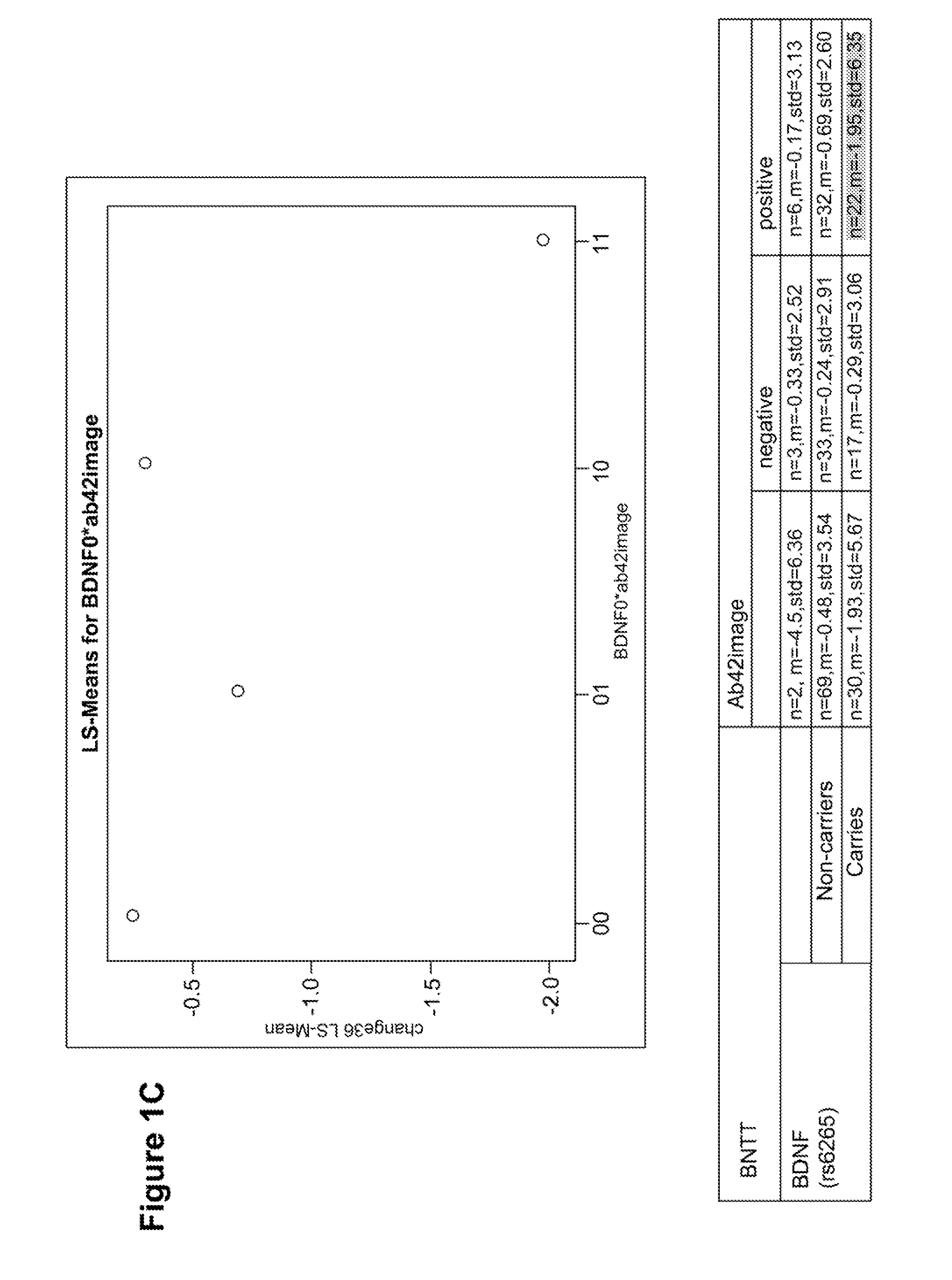
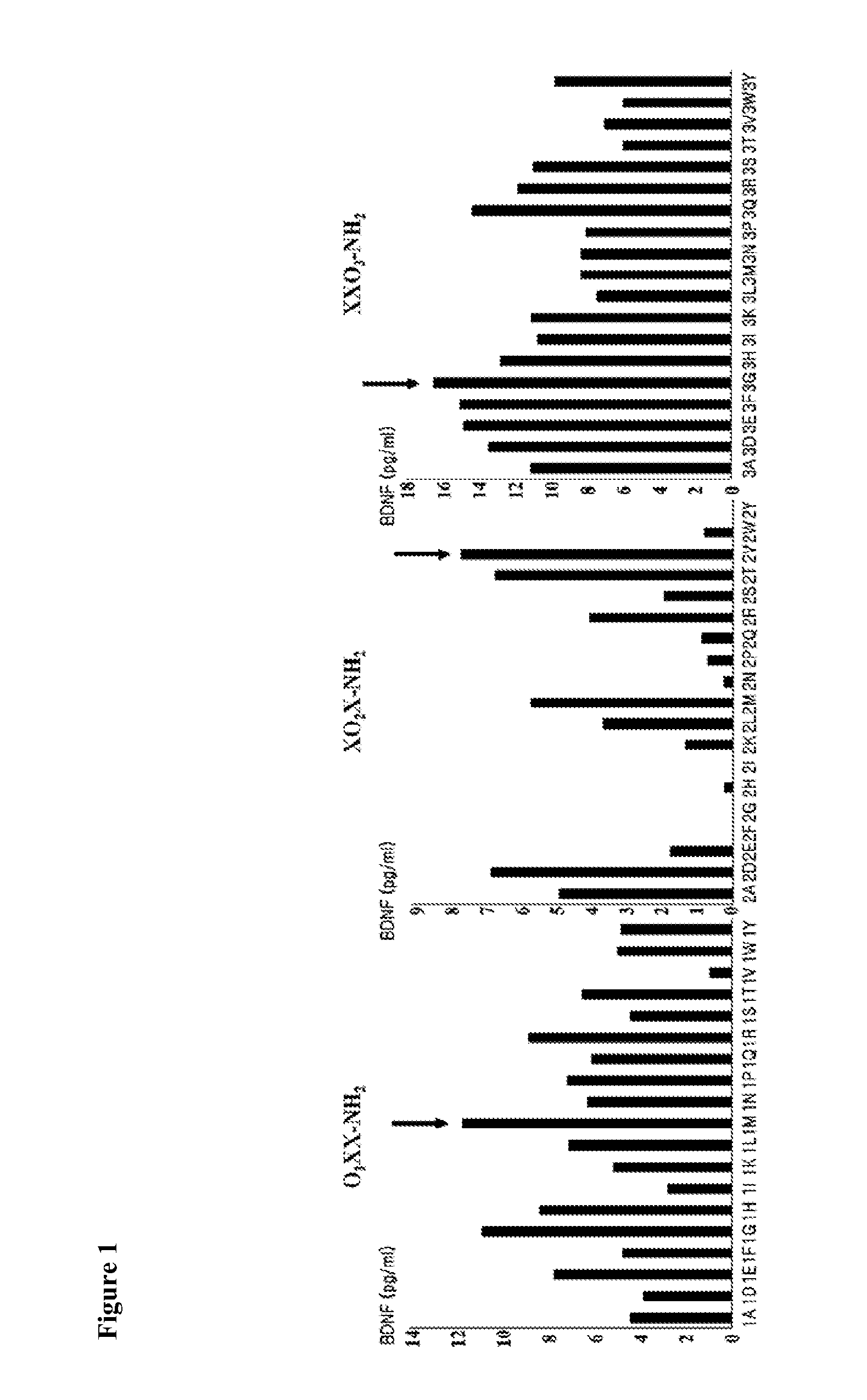
Genetic and image biomarkets associated with decline in cognitive measures and brain glucose metabolism in populations with alzheimer's disease or those susceptible to developing alzheimer's disease
InactiveUS20160177390A1Faster decline in brain glucose metabolismFaster 36 month cognitive declineMicrobiological testing/measurementLibrary screeningGenes mutationAmyloid beta
The present disclosure is based on the identification of biomarkers of combined genetic variants and imaging measurements, in predicting faster decline in cognitive measures and brain glucose metabolism in populations with Alzheimer's disease or those susceptible to developing Alzheimer's disease. The present disclosure provides a method of treating a patient with Alzheimer's disease (AD) or a subject susceptible to developing AD, comprising: (a) assaying a sample obtained from an early-stage AD patient or a subject susceptible to developing AD for the presence of a brain-derived neurotrophic factor (BDNF) gene mutation and / or a protein tyrosine phosphatase receptor-type, Z polypeptide 1 (Ptprz1) gene mutation; (b) determining whether the patient or subject is positive for brain amyloid-beta (Aβ), wherein the presence of brain Aβ in combination with the BDNF gene and / or Ptprz1 gene mutation correlates with a prediction of rapid cognitive decline; and (c) treating the patient or subject with early and aggressive therapy appropriate to treat AD with rapid cognitive decline.
Owner:BIOGEN INT NEUROSCI
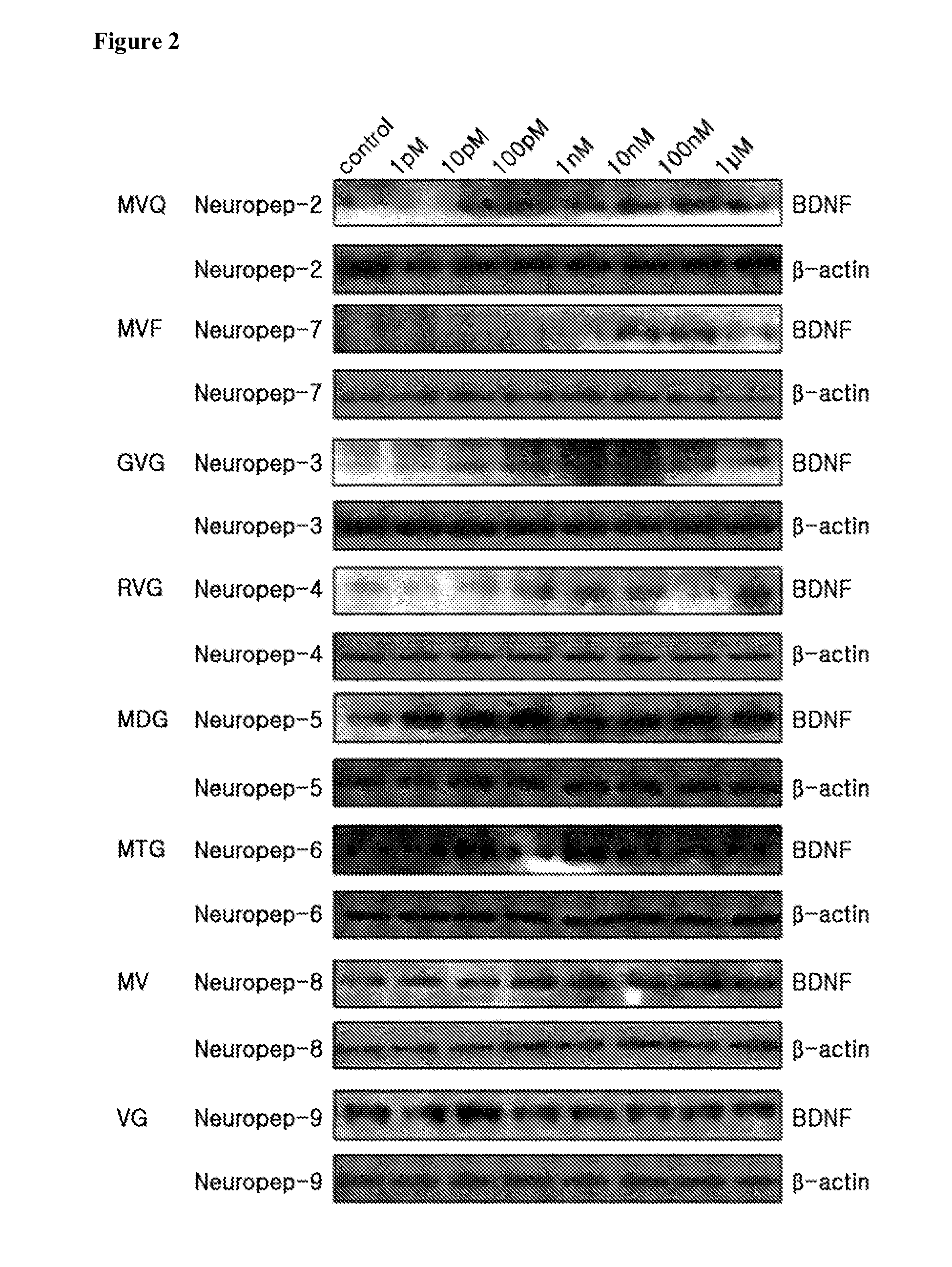
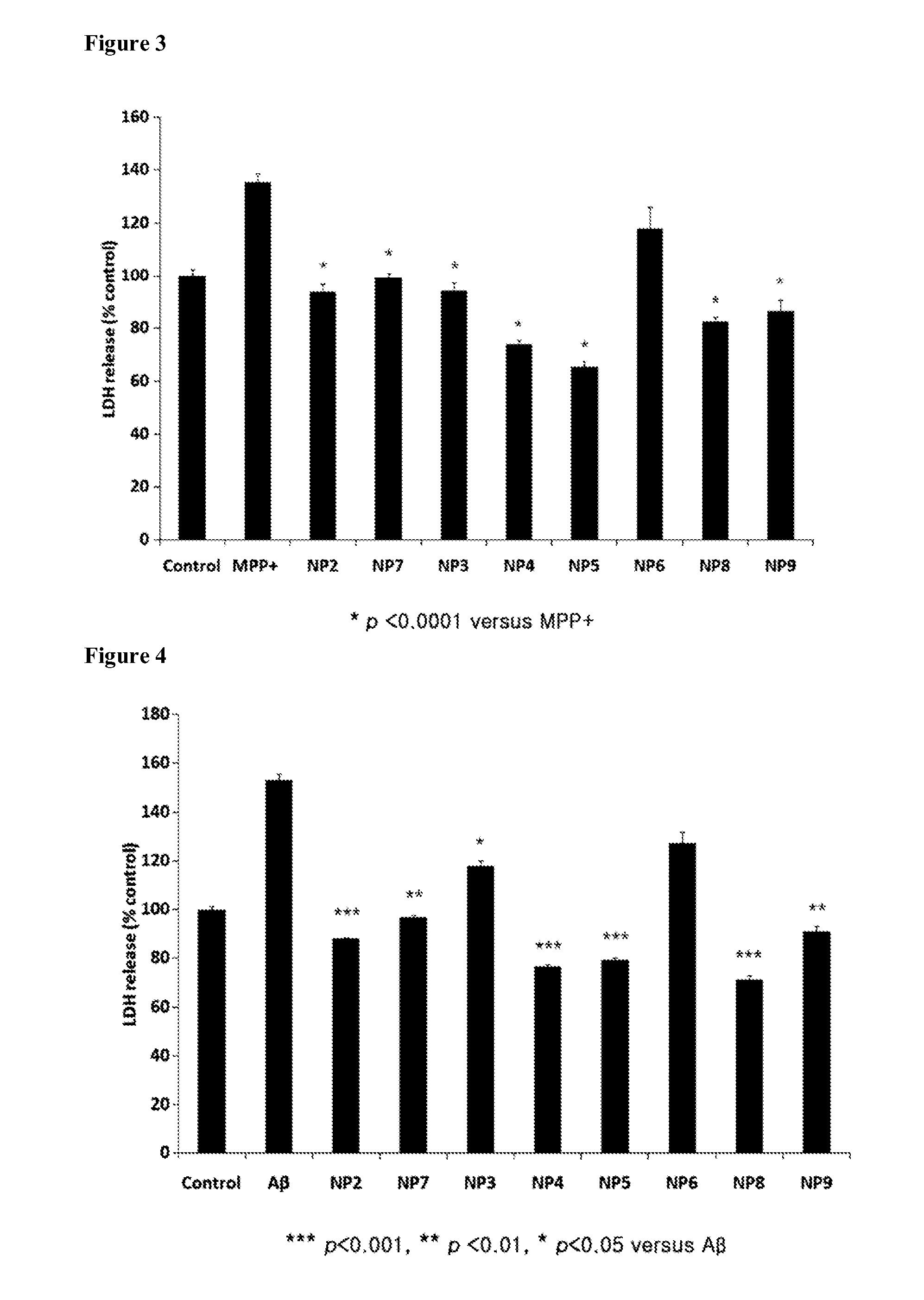
Novel peptide for augmenting and expression of bdnf and pharmaceutical composition for prevention and treatment of neurodegenerative diseases including alzheimer's disease or parkinson's disease, comprising the same
ActiveUS20120208747A1High expressionEasy passNervous disorderDipeptide ingredientsMedicineBrain-derived neurotrophic factor
Disclosed are peptides for augmenting the expression of BDNF (brain-derived neurotrophic factor) and a pharmaceutical composition for the prevention and treatment of Alzheimer's disease or Parkinson's disease, comprising the same. The peptides can induce the expression of BDNF in dopamine-reactive human cells, pass easily through the blood-brain barrier thanks to their low molecular weights and are almost free of cytotoxicity. Thus, they are useful in the prevention and treatment of neuropathies such as Alzheimer's disease or Parkinson's disease.
Owner:SUNGKYUNKWAN UNIVERSITY
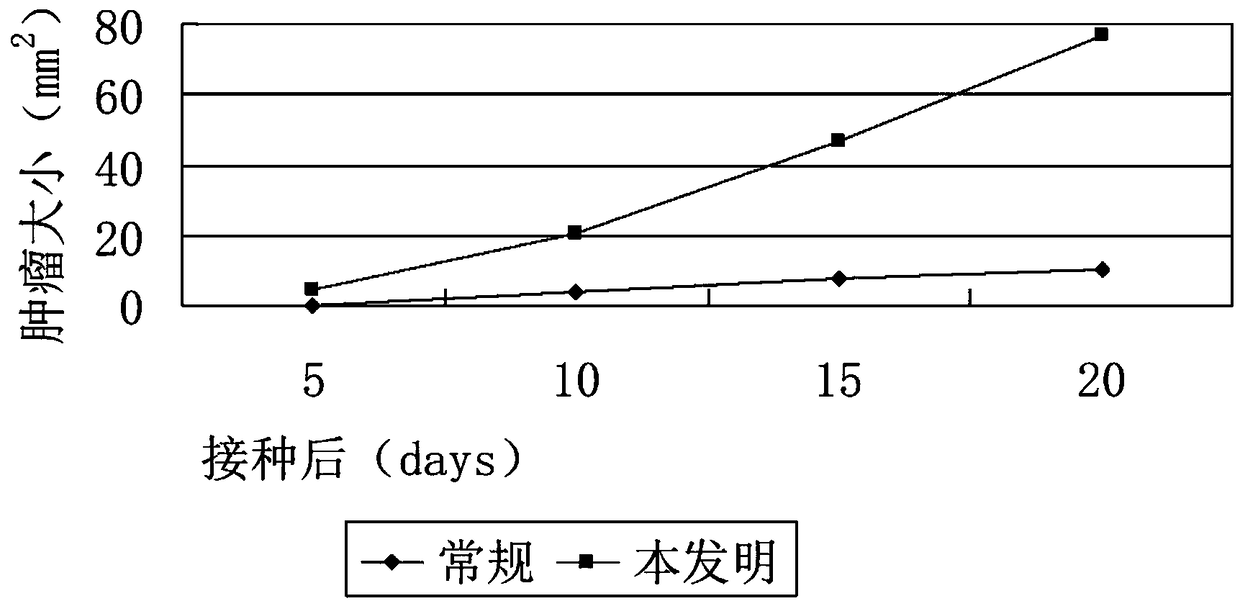
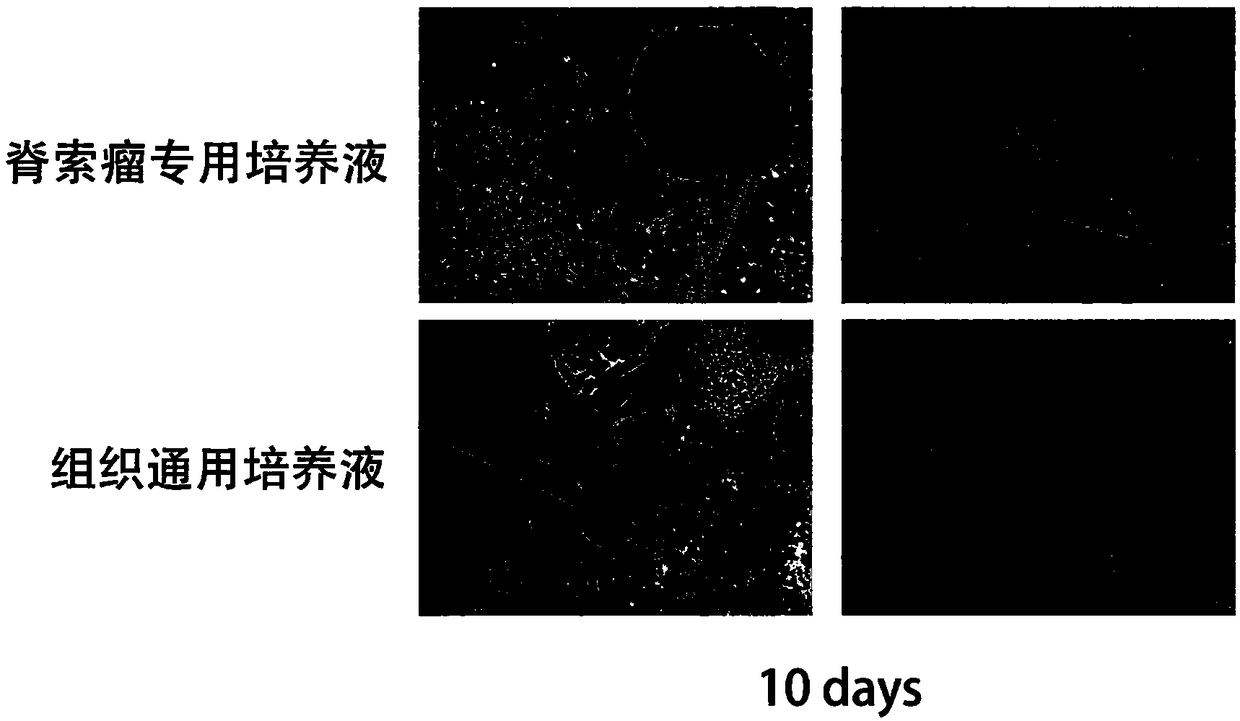
Tissue preserving fluid
ActiveCN108719274AImprove the shortcomings of unknown serum componentsHigh sex ratioDead animal preservationChordomaBrain-derived neurotrophic factor
The invention discloses tissue preserving fluid. Each 50mL of the tissue preserving fluid is prepared from the following components: 50ml of DMEM (Dulbecco's Modified Eagle Medium) / F12, 90 to 110mu Lof bFGF (Basic Fibroblast Growth Factor), 240 to 260mu L of N2, 480 to 520mu L of B27, 40 to 60mu L of Y-27632, 400 to 500ng of BDNF (Brain Derived Neurotrophic Factor) and 400 to 500ng of NT3. By applying the tissue preserving fluid disclosed by the invention, the activity of fresh tumor cells can be kept for relatively long time, so that the efficiency of obtaining a PDX (Patient-Derived tumor Xenograft) model is improved. Compared with convectional preserving fluid, the PDX model utilizing the preserving fluid has the advantage that the tumor formation rate is greatly increased and the tumor utilization rate is greatly improved; the tumor formation and primary cell culture time is remarkably shortened. Moreover, the method disclosed by the invention can be used for enhancing the activity of a chordoma sample, so that the cost of a chordoma PDX model experiment is reduced; precision medical treatment can be carried out on tumors of more patients through the PDX model, so that the possibility of healing chordoma is improved.
Owner:HUNAN SAIAOWEI BIOTECH CO LTD
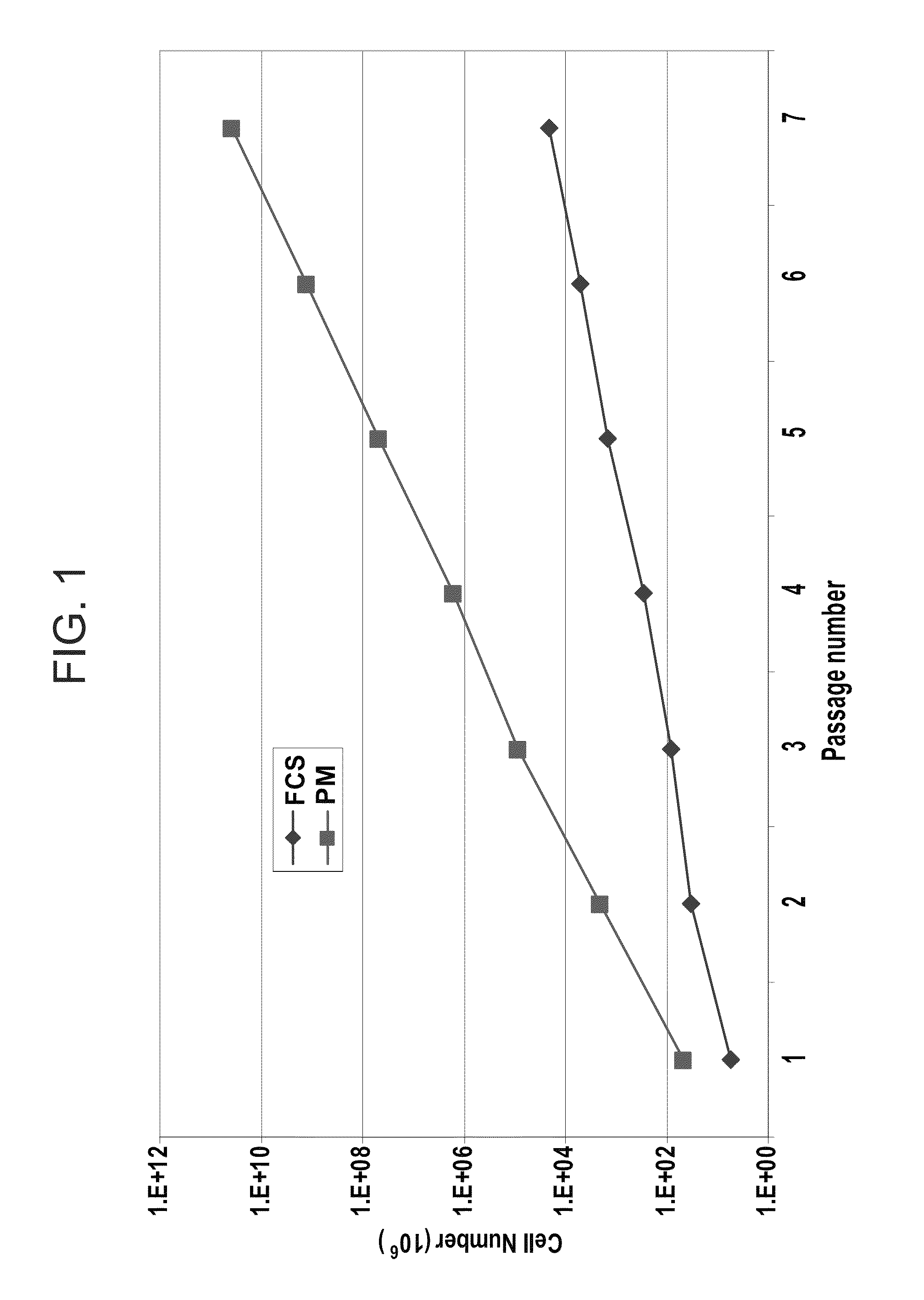
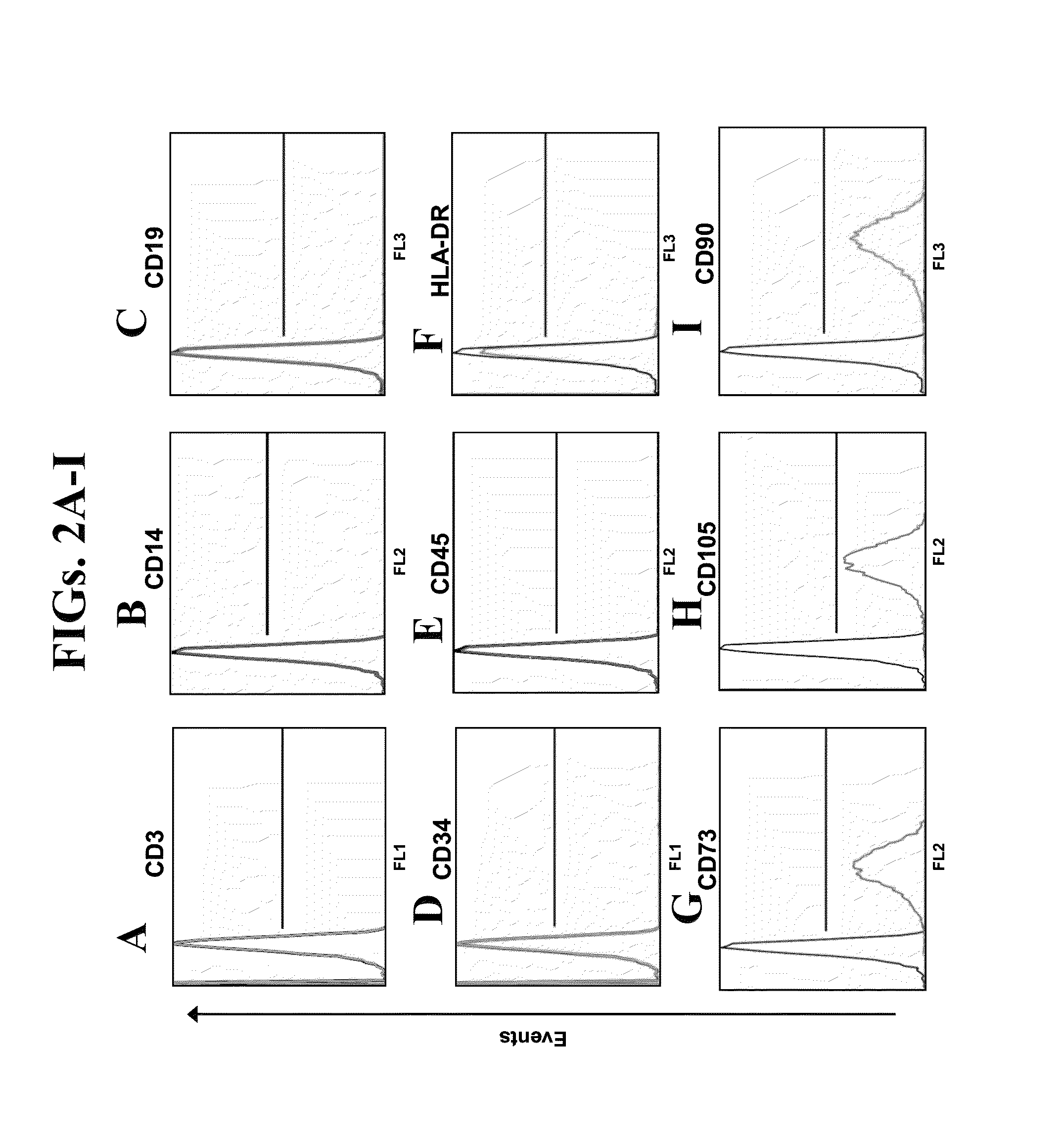
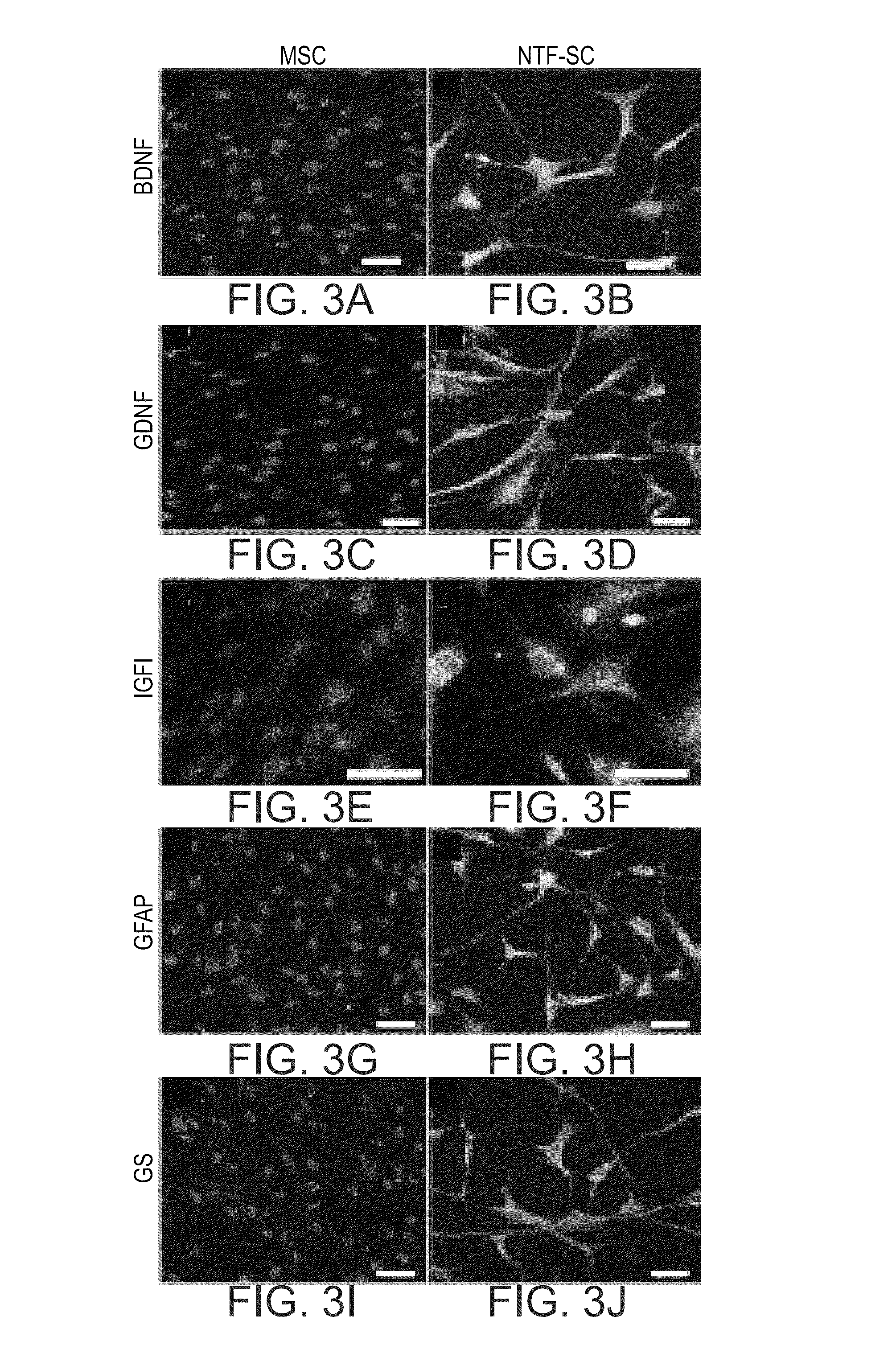
Mesenchymal stem cells for the treatment of CNS diseases
ActiveUS8663987B2Bone marrow stroma cellsNervous disorderCns diseaseBrain-derived neurotrophic factor
An isolated human cell is disclosed comprising at least one mesenchymal stem cell phenotype and secreting brain-derived neurotrophic factor (BDNF), wherein a basal secretion of the BDNF is at least five times greater than a basal secretion of the BDNF in a mesenchymal stem cell. Methods of generating same and uses of same are also disclosed.
Owner:BRAINSTORM CELL THERAPEUTICS LTD +1
Nerve repairing material and preparation method thereof
ActiveCN103933619AQuick combinationAvoid inactivationNervous disorderPeptide/protein ingredientsVascular endotheliumNerve repair
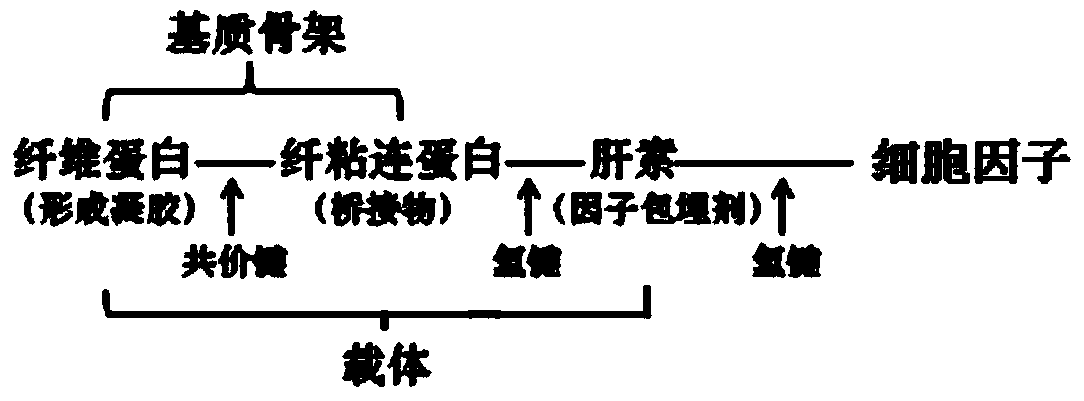
The invention discloses a nerve repairing material and a preparation method thereof. The nerve repairing material comprises a combination of cell factors capable of promoting nerve growth, and a controlled-release carrier of the cell factors, wherein the carrier is mainly composed of fibrinogen, fibronectin, heparin, fibrin stabilizing factor, thrombin and calcium chloride, wherein the combination of the cell factors capable of promoting nerve growth is optimally at least two of nerve growth factor, brain-derived neurotrophic factor, basic fibroblast growth factor and vascular endothelial cell growth factor, and is embedded in the carrier. According to the nerve repairing material, the speed of releasing cell factors can be regulated according to the nerve repairing progress by utilizing 'intelligent release' of the controlled-release carrier, so that the functions of cell factors can be progressively played; furthermore, the nerve repairing can be further promoted by utilizing the synergy of the cell factors.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Induction composition of human mesenchymal stem cells, induction differentiation culture liquid and in-vitro induction method and application
ActiveCN109694851ASmall side effectsIncrease contentNervous disorderPeptide/protein ingredientsSide effectNerve cells
The invention provides an induction composition of human mesenchymal stem cells, an induction differentiation culture liquid and an in-vitro induction method and an application, and relates to the field of nerve cell regeneration. The induction composition includes a brain-derived neurotrophic factor, a nerve growth factor, an epidermal cell growth factor, a fibroblast growth factor, a N2 cell culture additive, a B27 cell culture additive, rhodioloside and astragalus polysaccharide. In the induction composition provided by the invention, rhodioloside, astragalus polysaccharide and cytokines are compounded, induced differentiation nerve cells have the advantages of high content, strong activity, short period and low cost, can solve the problem of low differentiation efficiency when human mesenchymal stem cells are induced in vitro, improve the survival status of the induced nerve cells, and have little toxic and side effects on cells. The induction composition can be used for inducing the human mesenchymal stem cells, is easy to operate, enhances the activity of the differentiated nerve cells and shortens the induction differentiation time.
Owner:JILIN TUO HUA BIOTECH
Chitosan artificial nerve graft containing neurotrophic factor and preparation method thereof
InactiveCN102343112AImprove adsorption capacityEasy to transportProsthesisGlial cell line-derived neurotrophic factorCross-link
The invention discloses a chitosan artificial nerve graft containing a neurotrophic factor and a preparation method thereof. The chitosan artificial nerve graft is prepared by processing chitosan into a nerve conduit with a porous structure and high tensile strength by virtue of weak acid dissolution, mold injection, freezing formation, neutralization and immobilization, cleaning, lyophilization and other processes under a condition of not adding any foaming agent or cross linking agent. The cell factor with a treatment effect in the nerve conduit is one or more of a basic fibroblast growth factor (bFGF), a brain derived neurotrophic factor (BDNF), a neurotrophic factor 3 (NT-3), a basic fibroblast cell growth factor (bFGF) and a glial-cell-line derived neurotrophic factor (GDNF) and the like. The product provided by the invention contains the neurotrophic factor, adopts degradable materials, and has excellent biocompatibility with a human body. The prepared product does not contain exogenous toxic or side-effect substances brought by a preparation process.
Owner:JIANGSU YITONG BIOTECHNOLOGY CO LTD
Hyaluronic acid-orientated channel composite bracket material used for spinal cord injury repair
The invention discloses a hyaluronic acid- orientated channel composite bracket material used for spinal cord injury repair. The hyaluronic acid- orientated channel composite bracket material is composed of a hydrogel antibody releaser grafted with an NoGoR antibody and a factor-packaging poly lactic-co-glycolic acid copolymer microsphere which is injected into the hydrogel antibody releaser, wherein the inner part of the hydrogel antibody releaser grafted with the NoGoR antibody is shaped like a longitudinal channel which is in parallel arranged; the factor-packaging poly lactic-co-glycolic acid copolymer microsphere is shaped like a circular ball, and smooth in surface; the factor packaged by the polylactic acid-polyglycolic acid copolymer microsphere is a vascular endothelial growth factor and a brain-derived neurotrophic factor. The bracket material disclosed by the invention can improve a nerve regeneration microenvironment to promote spinal cord injury repair.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Culture medium for promoting differentiation of rat neural stem cells and use method of culture medium
ActiveCN104017771AHigh differentiation efficiencyNervous system cellsBrain-derived neurotrophic factorNeural Growth
The invention discloses a culture medium for promoting differentiation of rat neural stem cells and a use method of the culture medium. The culture medium contains B-27 Supplement without retinoic acid, L-Glutamine, FBS (fetal bovine serum), beta-NGF (neural growth factor), BDNF (brain derived neurotrophic factor), RA and vitamin E in Neurobasal-A medium. The culture medium is low in cost, the problem of low neural stem cell differentiation efficiency in the prior art is solved, and the efficiency of differentiating neural stem cells into nerve cells is remarkably improved.
Owner:CENT SOUTH UNIV
Brain-derived-neurotrophic-factor accelerant polypeptide and application
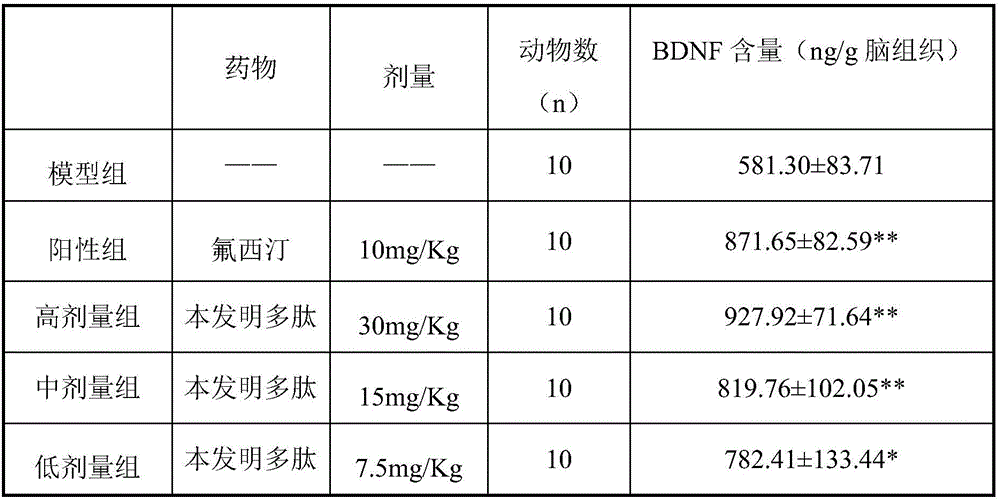
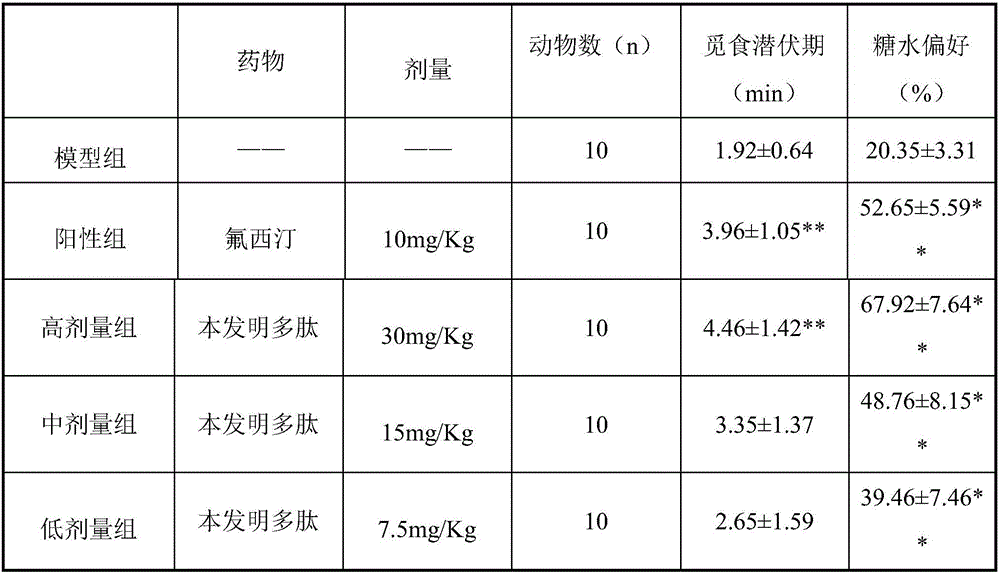
InactiveCN106366166AElevated BDNF contentIncrease intakePeptide-nucleic acidsNervous disorderBrain-derived neurotrophic factorIn vivo
The invention relates to the field of medicine, in particular to a polypeptide capable of simulating the brain-derived-neurotrophic-factor physiological function and reducing depressive symptoms. The sequence is SEQ ID NO:1, and the SEQ ID NO:1 is a bran-new sequence. The polypeptide has the advantages that the BDNF content of chronic inscrutable stress model rat brain tissue can be increased. The in-vivo pharmacological result shows that depressive symptoms of acute and chronic depression model animals can be improved through the polypeptide, and the polypeptide can serve as an effective candidate treating method for clinical depression.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com