Application of phthalide compound
A compound, phthalide technology, applied in the application field of phthalide compounds, can solve the problem of no significant curative effect on motor neuron degenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
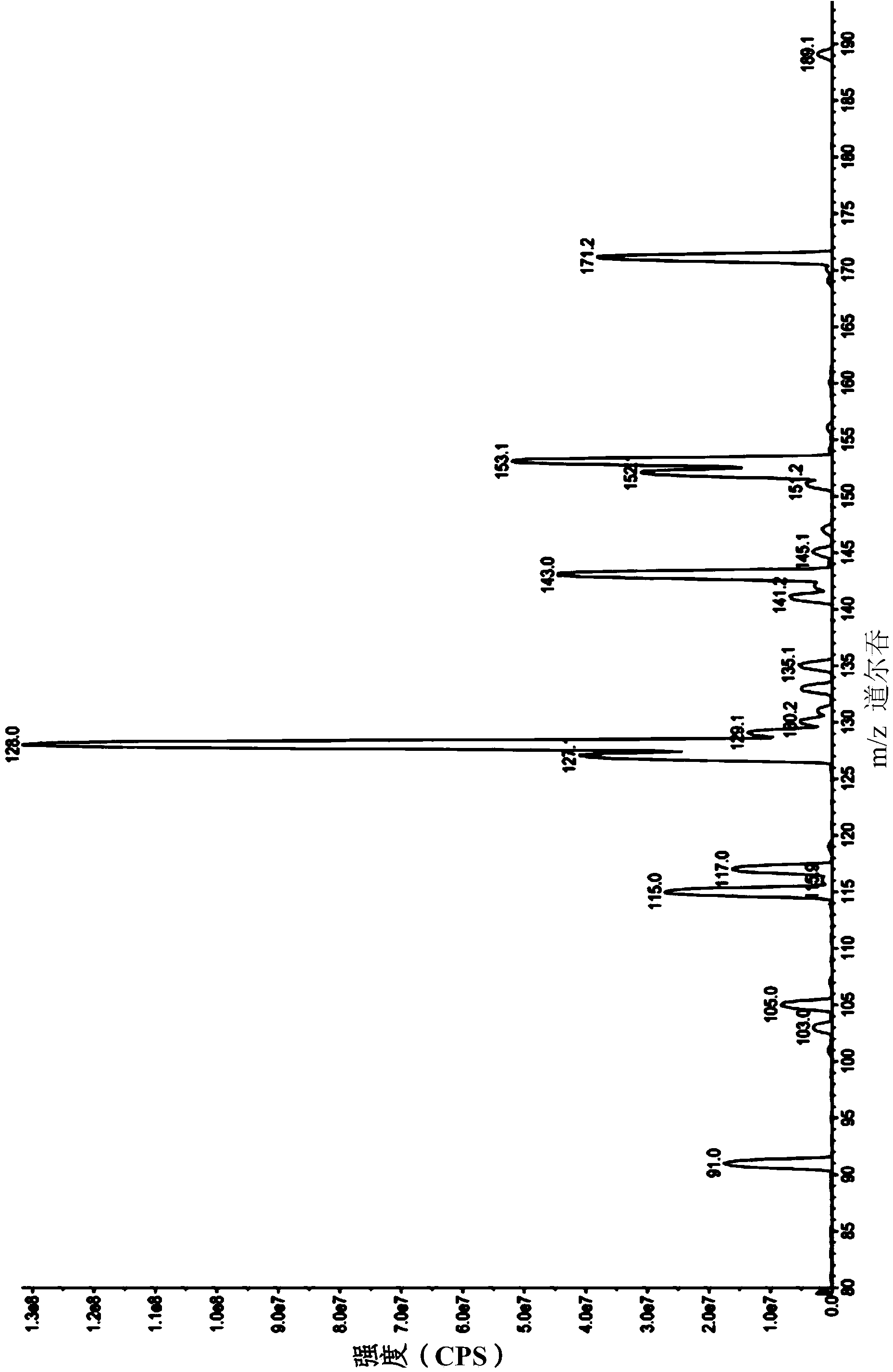
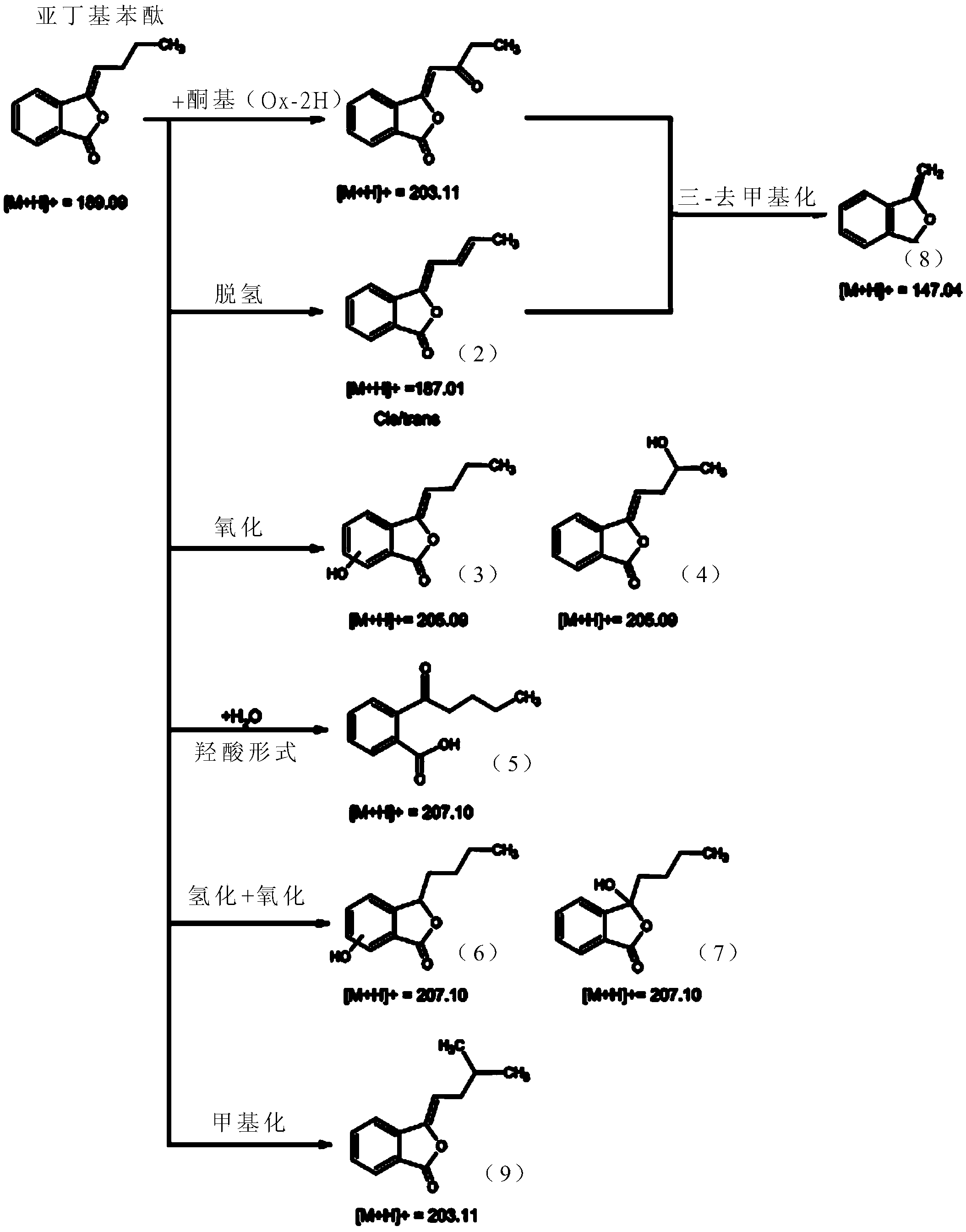
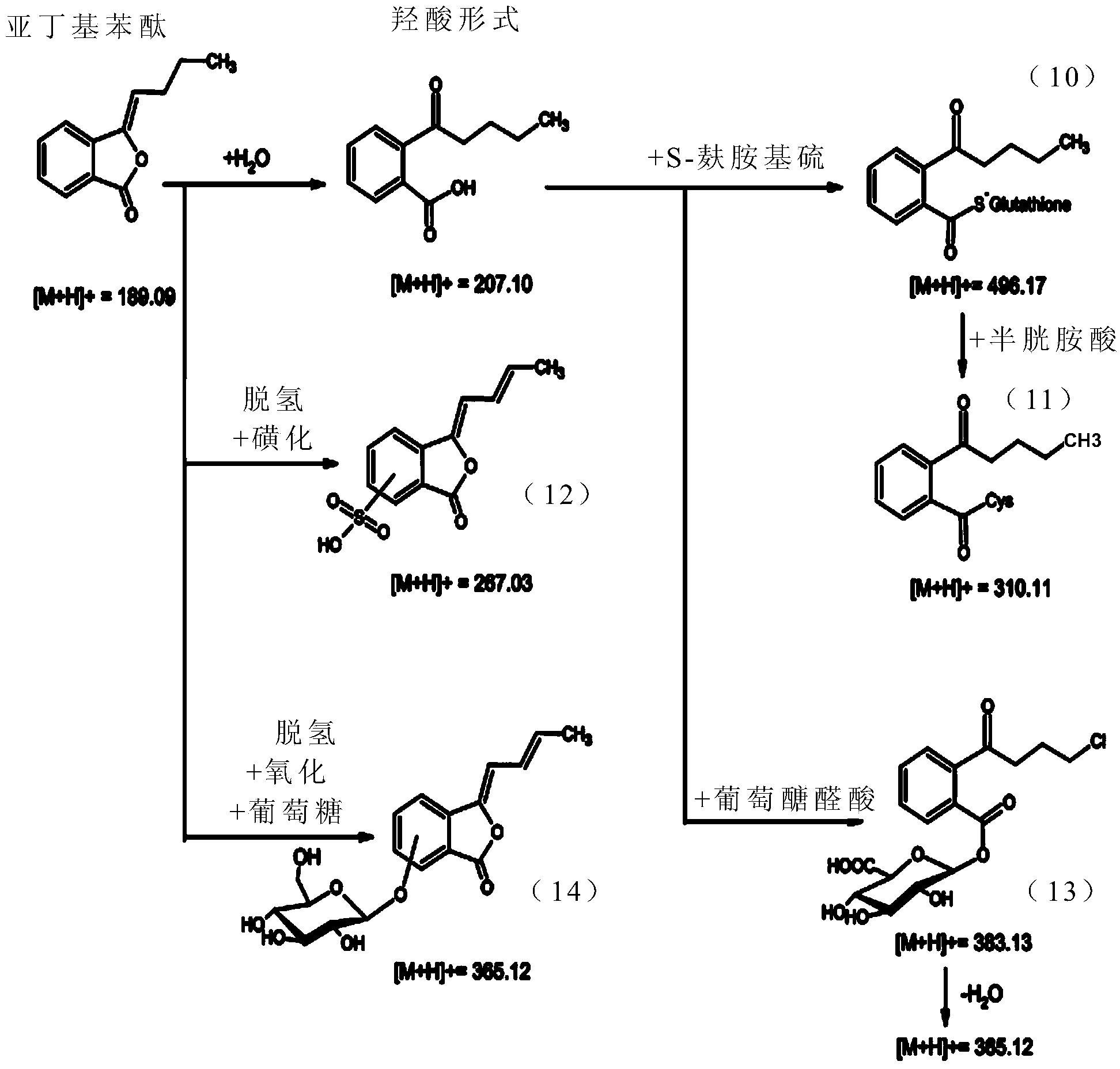
[0059] [Example 1] Metabolites of Butylenephthalide
[0060] It is known that the drug metabolism pathways of drugs in the liver of living organisms can be mainly divided into first-order (phase I) metabolism and second-order (phase II) metabolism. The first-order metabolism is mainly the oxidation-reduction reaction or hydrolysis reaction of the drug , the second-order metabolism is mainly carried out by the cytochrome P450 monooxygenase (cytochrome P450 (CYP450) monooxygenase) system for metabolic reactions. In this example, the mixed reaction of butylenephthalide with liver microsomes or cryopreserved liver cells (cryopreserved hepatocytes) in vitro is used to simulate the process of butylenephthalide in the liver of a living body. The first-order metabolism and second-order metabolism of phthalide, and the products in the solution after the reaction were analyzed by liquid chromatography tandem mass spectrometry (LC-MS / MS) to confirm the metabolites and metabolic profiles of...
Embodiment 2
[0074] [Example 2] In vivo test of survival rate of transgenic mice
[0075] It is known that about 20% of the patients with amyotrophic lateral sclerosis will have the gene mutation of copper / zinc superoxide dismutase (SODl), among which the G93A mutation site is the most. The mutant human SOD1-G93A gene-transformed mouse (herein referred to as "SOD1-G93A gene-transformed mouse") can be used clinically for the study of amyotrophic lateral sclerosis because of its similar disease characteristics to humans. As an animal model of the disease, the SOD1-G93A gene transfected mice will start to develop symptoms at about the 90th ± 5th day after birth, and die at about the 125th ± 5th day after birth.
[0076] In this example, the above-mentioned SOD1-G93A gene transfected mice were used as research objects for in vivo experiments. The experiments were divided into the following five groups: (A) control group (no drug administration); (B) Relidex Experimental group: On the 60th day...
Embodiment 3
[0081] [Example 3] In vivo test of atrophic lateral sclerosis
[0082]In this example, SOD1-G93A gene transfected mice were used as the subjects of in vivo experiments. The 60-day-old SOD1-G93A gene transgenic mice were randomly divided into the following five groups: (A) control group (no drug administration); (B) Relidex experimental group: born in SOD1-G93A gene transgenic mice On the 60th day after that, the regular intraperitoneal injection of Rilide was started, once a day, and each dose was 16 mg / kg body weight; (C) Butylidenephthalide (n-butylidenephthalide: containing Z-BP95%+E-BP5%) experimental group (BP500mg / kg / qd): On the 60th day after birth, the SOD1-G93A gene transfected mice were orally administered with butylenephthalide (purchased from Jingming Chemical), once a day, each dose of 500 mg / kg body weight ; (D) Butylidenephthalide (n-butylidenephthalide: containing Z-BP95%+E-BP5%) experimental group (BP250mg / kg / bid): start timing on the 60th day after birth of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com