Brain-derived-neurotrophic-factor accelerant polypeptide and application
A neurotrophic factor and enhancer technology, applied in nervous system diseases, peptides, depsipeptides, etc., can solve the problems of large molecular weight and reduce drug efficacy, so as to improve the intake of sugar water, improve depression symptoms, and reduce foraging latency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
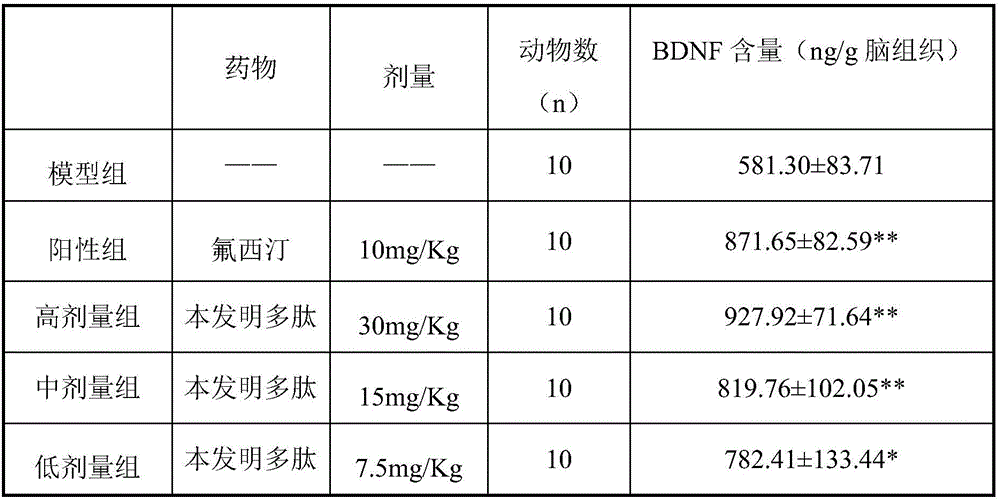
[0015] Effect of brain-derived neurotrophic factor promoter polypeptide on BDNF content in brain tissue of chronic unpredictable stress model rats.
[0016] SD rats were used as test animals, and 60 rats, half male and half male, with a body weight of 180-220 g, were randomly divided into 6 groups, high-dose group, middle-dose group, low-dose group, positive control group, model group, Blank group, each group l0. Establish a rat chronic unpredictable stress model: the stress content is: restraint for 4 hours; wet bedding overnight; shaking for 1 hour (160r / min); fasting for 12 hours; Clamp the tail for 1 min; crowd overnight (8 / cage). Two stresses were used during the day, and one stress was used at night, and the stress was continued for 4 weeks. Administer after the last stimulation. Dosing regimen: the treatment group was subcutaneously injected with the polypeptide of the present invention at doses of 30, 15, and 7.5 mg / Kg, the control group was given fluoxetine at a do...
Embodiment 2
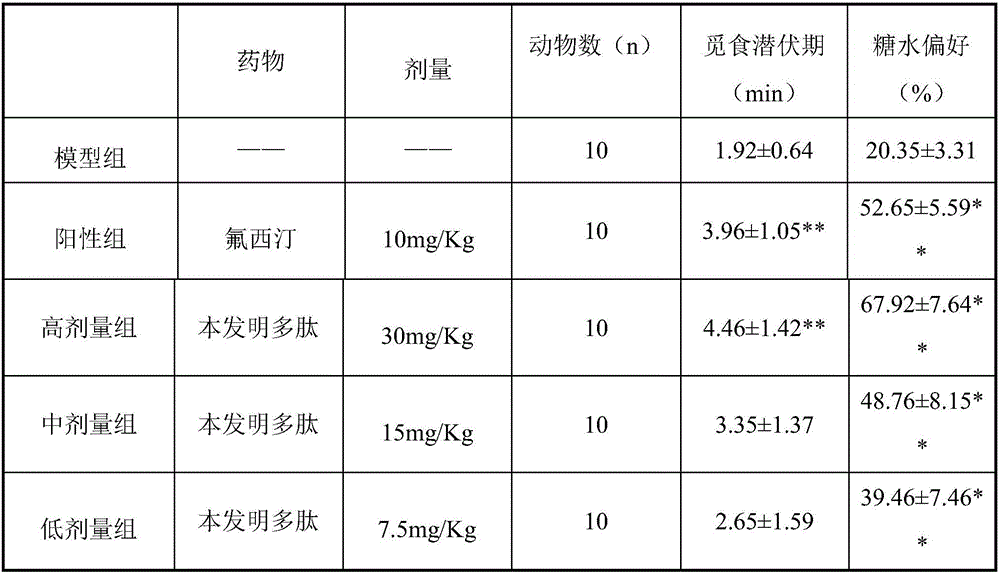
[0022] Effects of brain-derived neurotrophic factor-promoter peptides in a mouse forced-swim stress-despair model.
[0023] Using ICR mice as the test animals, 50 mice, half male and half male, weighing 18-22g, were randomly divided into 5 groups: high-dose group, middle-dose group, low-dose group, positive control group, blank group, Each group l0. The treatment group was subcutaneously injected with the polypeptide of the present invention at doses of 30, 15, and 7.5 mg / Kg, the control group was given fluoxetine at a dose of 10 mg / Kg, and the blank group was given normal saline for 7 consecutive days. One hour after administration on the seventh day, the mice were subjected to a forced swimming test. The mice were forced to swim in water at 20-25°C for 6 minutes, and the immobility time within 4 minutes after recording was recorded. The time spent floating with the head out of the water is used to evaluate the effect of the polypeptide of the present invention on the model ...
Embodiment 3
[0029] Effects of brain-derived neurotrophic factor-promoter peptides in a mouse tail-suspension stress hopelessness model.
[0030] Using ICR mice as the test animals, 50 mice, half male and half male, weighing 18-22g, were randomly divided into 5 groups: high-dose group, middle-dose group, low-dose group, positive control group, blank group, Each group l0. The treatment group was subcutaneously injected with the polypeptide of the present invention at doses of 30, 15, and 7.5 mg / Kg, the control group was given fluoxetine at a dose of 10 mg / Kg, and the blank group was given normal saline for 7 consecutive days. 1h after administration on the 7th day, the mice were subjected to a tail-suspension test, and the mice were fixed with adhesive tape at a place about 1 cm from the end of their tails, and the mice were kept in a suspended state for 6 minutes, and the immobility time of 4 minutes after recording was used to evaluate this product. Effects of Invention Peptides on a Mou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com