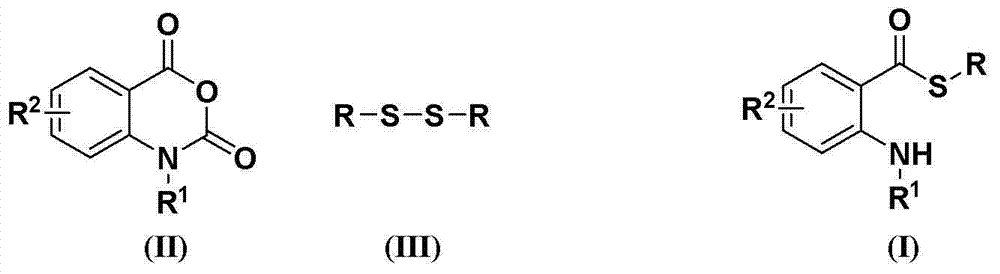
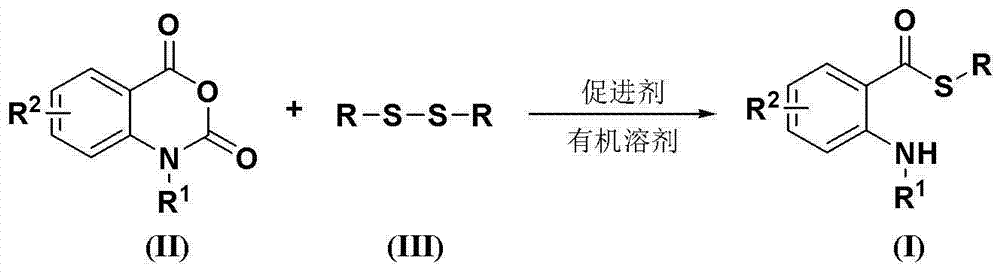
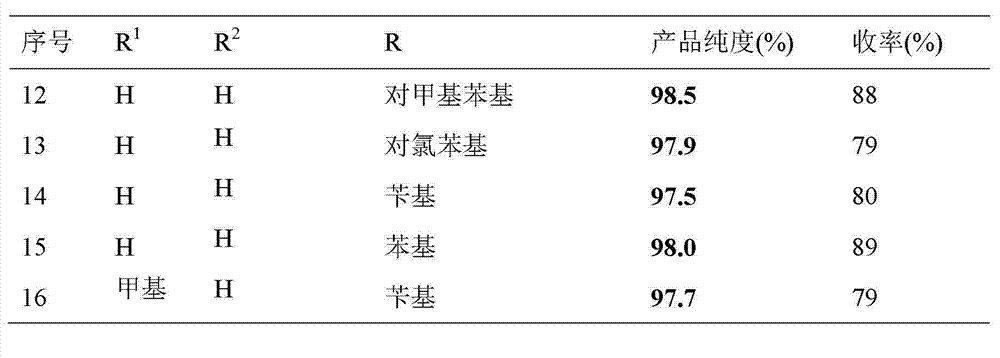
Synthesis method of S-substituted-anthranilate thioester derivatives
A technology of aminobenzoic acid thioester and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of high risk factor, limited promotion, high volatility, etc., and achieve the effects of environmental friendliness, simple and safe operation, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Disulfide, isatoic anhydride compounds, accelerators and basic compounds are fed in a molar ratio of 1.0:2.5:4.0:3.0, the disulfide is diphenyl disulfide, and the feeding quality is 21.8g (0.1mol); The red acid anhydride compound is isatoic anhydride, and the feeding quality is 40.7g (0.25mol); the accelerator is sodium dithionite, and the feeding quality is 69.6g (0.4mol); the basic compound is potassium carbonate, and the feeding quality is 41.4g (0.3mol); The organic solvent is 218 g of dimethyl sulfoxide, and its total consumption is 10 times of the mass of diphenyl disulfide.
[0032] Dissolve isatoic anhydride and accelerator in an organic solvent (the amount of organic solvent is 6 times the mass of diphenyl disulfide). Dissolve diphenyl disulfide and potassium carbonate in an organic solvent (the amount of organic solvent is 4 times the mass of diphenyl disulfide), slowly add dropwise to the solution of isatoic anhydride and accelerator, and the reaction tempera...
Embodiment 2
[0036] Disulfide, isatoic anhydride compounds, accelerators and basic compounds are fed in a molar ratio of 1.0:2.5:4.0:3.0, the disulfide is diphenyl disulfide, and the feeding quality is 21.8g (0.1mol); The red acid anhydride compound is isatoic anhydride, and the feeding mass is 40.7g (0.25mol); The solvent is 218 g of dimethyl sulfoxide, and its total consumption is 10 times the mass of diphenyl disulfide.
[0037]All the other are the same as in Example 1, the resulting product S-phenylanthranilic acid thioester 14.2g, yield 62%, purity 97.5%.
Embodiment 3
[0039] Disulfide, isatoic anhydride compounds, accelerators and basic compounds are fed in a molar ratio of 1.0:2.5:4.0:3.0, the disulfide is diphenyl disulfide, and the feeding quality is 21.8g (0.1mol); The red acid anhydride compound is isatoic anhydride, and the feeding quality is 40.7g (0.25mol); the accelerator is sodium dithionite, and the feeding quality is 69.6g (0.4mol); the basic compound is potassium carbonate, and the feeding quality is 41.4g (0.3mol); The organic solvent is 218 g of N,N,-dimethylformamide, the total amount of which is 10 times the mass of diphenyl disulfide.
[0040] The rest are the same as in Example 1, and the resulting product S-phenyl anthranilate thioester is 17.9g, with a yield of 78% and a purity of 97.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com