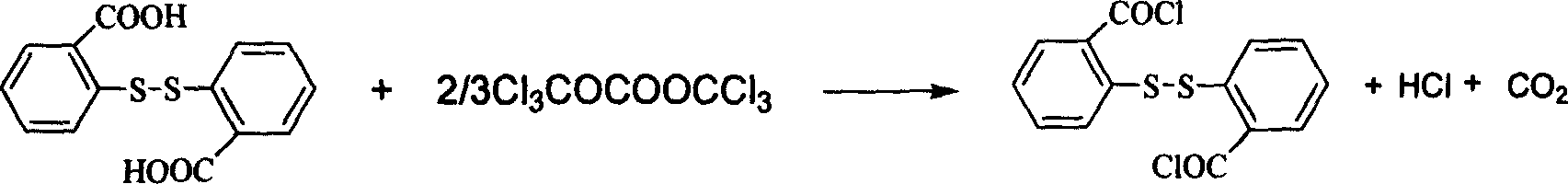2,2'-di-thio-bibenzoyl cholride chemical synthesis method
A technology of dithiobisbenzoyl chloride and dithiobisbenzoic acid, applied in 2 fields, can solve the problems of high sealing requirements of reaction equipment, low product yield and purity, and high production cost, and achieves high implementation value and Social and economic benefits, low production cost, and no three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The molar ratio of 2,2'-dithiobisbenzoic acid:bis(trichloromethyl)carbonate:catalyst is 1:0.7:0.01, and the catalyst is 1,3-dimethyl-2-imidazolidinone, organic The solvent is toluene, and its consumption is 3 times of the mass of 2,2'-dithiobisbenzoic acid.
[0016] In a 250mL four-necked flask equipped with a thermometer, reflux condenser, constant pressure dropping funnel and mechanical stirring, add 30.6g (100mmol) of 2,2'-dithiobisbenzoic acid, bis(trichloromethyl)carbonic acid 20.8 g (70 mmol) of ester, 92 g of toluene and 0.1 g (1 mmol) of catalyst. After the addition, the temperature was raised to 45°C, and the reaction was carried out at 45-50°C for 8 hours, monitored by TLC (developing solvent: ethyl acetate:methanol=2:1), after the reaction was completed, the solvent was distilled off under reduced pressure to obtain 2,2' The crude product of dithiobisbenzoyl chloride was recrystallized to obtain 32.6 g of light yellow 2,2'-dithiobisbenzoyl chloride crystals,...
Embodiment 2
[0018] Feeding molar ratio 2,2'-dithiobisbenzoic acid: bis(trichloromethyl) carbonate: catalyst is 1: 1.0:0.01, 2,2'-dithiobisbenzoic acid charging capacity is 30.6g ( 100mmol), two (trichloromethyl) carbonate charging capacity is 29.7g (100mmol), catalyzer is 1,3-dimethyl-2-imidazolidinone, and its consumption is 0.1g (1mmol), and organic solvent is toluene , and its dosage is 92g, that is, 3 times the mass of 2,2'-dithiobisbenzoic acid.
[0019] The reaction temperature is 85-90° C., and the reaction time is 4 hours. Other operations are the same as in Example 1. The product yield is 90.0%, the purity is 98.4%, and the melting point is 152-153° C. 1 H NMR (CDCl 3 )δ: 8.4(m, 2H), 7.7(d, 2H), 7.5(m, 2H), 7.3-7.4(m, 2H).
Embodiment 3
[0021] Feeding molar ratio 2,2'-dithiobisbenzoic acid: bis(trichloromethyl) carbonate: catalyst is 1: 1.6:0.01, 2,2'-dithiobisbenzoic acid charging capacity is 30.6g ( 100mmol), two (trichloromethyl) carbonate charging capacity is 47.5g (160mmol), and catalyst is 1,3-dimethyl-2-imidazolidinone, and its consumption is 0.1g (1mmol), and organic solvent is toluene , and its dosage is 92g, that is, 3 times the mass of 2,2'-dithiobisbenzoic acid.
[0022] The reaction temperature is 108°C, the reaction time is 1 hour, other operations are the same as in Example 1, the product yield is 92.5%, the purity is 99.0%, and the melting point is 148-150°C. 1 H NMR (CDCl 3 )δ: 8.4(m, 2H), 7.7(d, 2H), 7.5(m, 2H), 7.3-7.4(m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com