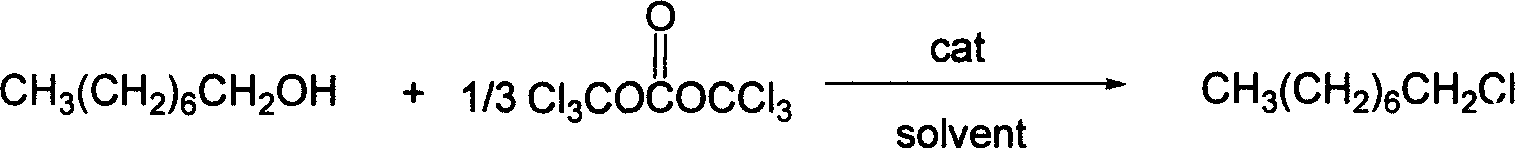Synthesis process of 1-chloro octane
A synthesis method and technology of chlorooctane, applied in the field of synthesis of 1-chlorooctane, can solve the problems of inconvenient and accurate measurement of phosgene, difficulty in storage and transportation, pollution, etc., achieve great implementation value and social and economic benefits, eliminate The effect of great safety hazards and advanced process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The amount ratio of feed material n-octanol: two (trichloromethyl) carbonates: organic amine catalyst is 1: 0.36: 0.2, and the feeding amount of n-octanol is 6.5g (0.05mol), two (trichloromethyl) The charging amount of carbonate is 5.35g (0.018mol), the organic solvent is chlorobenzene, and the consumption is 0.5 times of n-octanol quality, and the organic amine catalyst is triethylamine, and the consumption is 1.01g (0.01mol).
[0021] Dissolve bis(trichloromethyl)carbonate in the above-mentioned chlorobenzene, transfer to the constant pressure dropping funnel at low temperature, then transfer n-octanol to another constant pressure dropping funnel after diluting n-octanol with the same solvent At 60-110°C, slowly add it dropwise to a solution of triethylamine diluted with chlorobenzene, and keep this temperature for 4-9 hours. The reaction solution was naturally cooled to room temperature, neutralized with an alkaline solution to neutrality, then washed 3 times with ta...
Embodiment 2
[0023] The amount ratio of the feed material is n-octanol: two (trichloromethyl) carbonate: catalyst is 1: 0.4: 0.4, and the feeding amount of n-octanol is 6.5g (0.05mol), two (trichloromethyl) carbonate The feeding amount is 5.94g (0.02mol), the organic solvent is chlorobenzene, and the consumption is 0.3 times of n-octanol mass, and the catalyst is triethylamine, and the consumption is 2.02g (0.02mol).
[0024] Reaction temperature is 67~72 ℃, other operation is the same as embodiment 1, obtains product 5.58g, product yield 75.1%, purity 96.0%, boiling point 182~186 ℃.
Embodiment 3
[0026] The amount ratio of feed material is n-octanol: two (trichloromethyl) carbonate: catalyst is 1: 0.36: 0.6, and the feeding amount of n-octanol is 6.5g (0.05mol), two (trichloromethyl) carbonate The feeding amount is 5.35g (0.018mol), the organic solvent is chlorobenzene, the catalyst is triethylamine, and the consumption is 3.04g (0.03mol).
[0027] Reaction temperature is 80~85 ℃, other operation is the same as embodiment 1, obtains product 6.48g, product yield 87.2%, purity 98.4%, boiling point 182~186 ℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com