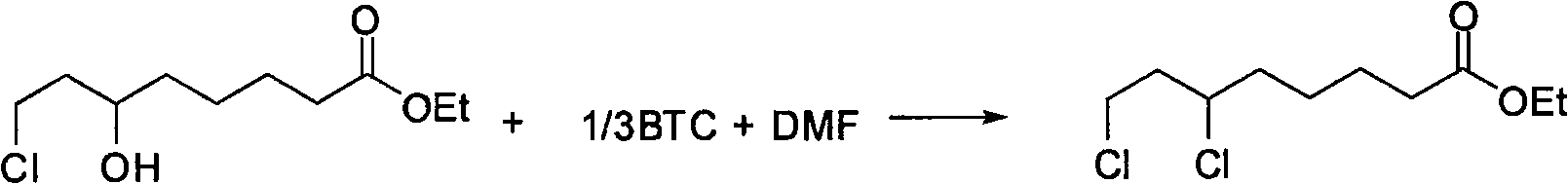Chemical method for synthesizing 6,8-dichloro ethyl cacodylic acid caprylate
A technology of ethyl dichlorooctanoate and ethyl chlorooctanoate, applied in 6 fields, can solve the problems of high sealing requirements of reaction equipment, difficult handling, large energy consumption, etc., and achieves high implementation value, social and economic benefits, and reasonable process conditions. , The effect of simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The ratio of the amount of feed material to 6-hydroxyl-8-chlorooctanoic acid ethyl ester: bis(trichloromethyl) carbonate: N, N-dimethylformamide is 1: 0.34: 1.2, and the dissolved bis(trichloromethyl) The organic solvent of carbonate is toluene, and its consumption is about 2 times of bis(trichloromethyl)carbonate quality.
[0021] In a 250mL three-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, dissolve 44.5g (200mmol) of ethyl 6-hydroxy-8-chlorooctanoate in 17.5g (240mmol) of N,N-dimethylformamide , add dropwise the organic solution of bis(trichloromethyl)carbonate (20.2g (68mmol) of bis(trichloromethyl)carbonate is dissolved in 40g toluene to prepare) under ice-water bath and stirring, and slowly warming up to 50°C, and react at 50-55°C for 8 hours, cool to below 30°C, neutralize with lye to neutrality, evaporate the solvent at normal pressure, and collect the distillate at 172-176°C at a vacuum of 5 mm Hg to obtain 6,8 -Ethyl dichl...
Embodiment 2
[0023] The ratio of the amount of feed material to 6-hydroxyl-8-chlorooctanoic acid ethyl ester: bis(trichloromethyl) carbonate: N, N-dimethylformamide is 1: 0.4: 1.2, 6-hydroxyl-8-chlorooctanoic acid Ethyl ester charging capacity is 44.5g (200mmol), two (trichloromethyl) carbonate charging capacity is 23.74g (80mmol), N, N-dimethylformamide charging capacity is 17.5g (240mmol), dissolving bis( The organic solvent of trichloromethyl) carbonate is toluene, and its consumption is about 1.5 times of two (trichloromethyl) carbonate quality.
[0024] The reaction temperature was 55-60° C., the reaction time was 7 hours, and other operations were the same as in Example 1 to obtain 44.4 g of ethyl 6,8-dichlorooctanoate with a molar yield of 92.2% and a purity of 98.2%.
Embodiment 3
[0026] The ratio of the amount of feed material to 6-hydroxyl-8-chlorooctanoic acid ethyl ester: bis(trichloromethyl) carbonate: N, N-dimethylformamide is 1: 0.4: 1.2, 6-hydroxyl-8-chlorooctanoic acid Ethyl ester charging capacity is 44.5g (200mmol), two (trichloromethyl) carbonate charging capacity is 23.7g (80mmol), N, N-dimethylformamide charging capacity is 17.5g (120mmol), dissolving bis( The organic solvent of trichloromethyl) carbonate is toluene, and its consumption is about 2 times of two (trichloromethyl) carbonate quality.
[0027] The reaction temperature was 70-75° C., and the reaction time was 6 hours. Other operations were the same as in Example 1 to obtain 45.6 g of ethyl 6,8-dichlorooctanoate with a molar yield of 94.7% and a purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com