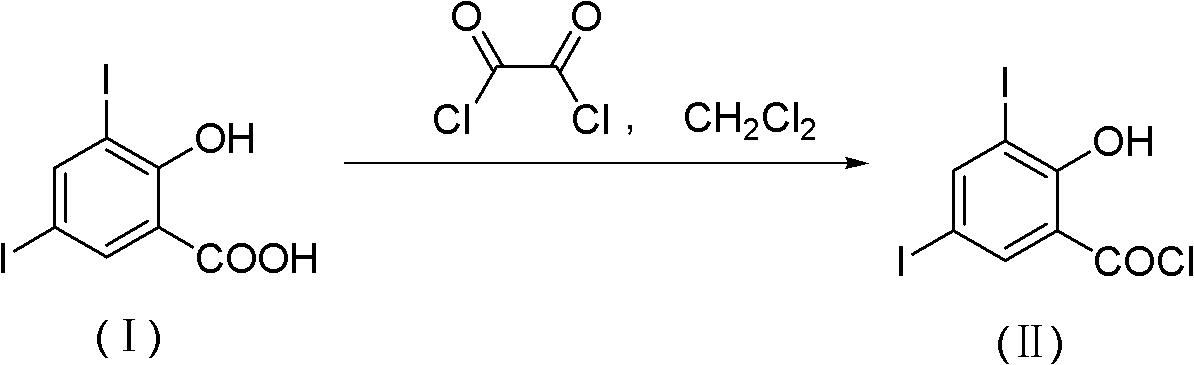
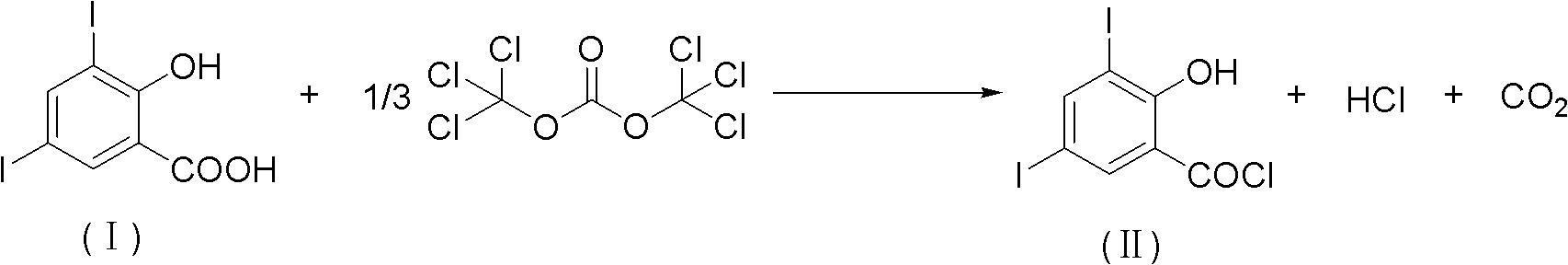
Method for chemically synthesizing 3,5-diio-2-hydroxybenzene formyl chloride
A technology for hydroxybenzoyl chloride and chemical synthesis, which is applied in 3 fields, can solve the problems of high energy consumption in the concentration process, difficult treatment of acidic wastewater, and large hidden dangers in production safety, and achieves low cost, high product yield and purity, and reasonable conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The ratio of the amount of 3,5-diiodo-2-hydroxybenzoic acid and bis(trichloromethyl) carbonate feed material is 1: 0.34, 3,5-diiodo-2-hydroxybenzoic acid and catalyst are 1: 0.01, the catalyst is pyridine, the organic solvent is toluene, and the mass ratio of the organic solvent to 3,5-diiodo-2-hydroxybenzoic acid is 5:1.
[0028] In a 500mL three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 39.0g (100mmol) of 3,5-diiodo-2-hydroxybenzoic acid and 10.1g (34mmol) of bis(trichloromethyl)carbonate , toluene 224.9mL (195.0g) and pyridine 0.1g (1mmol), warming up to 60 ℃ and stirring reaction for 8h, thin-layer chromatography tracking reaction (developing agent is petroleum ether: ethyl acetate=5: 1 (volume ratio)), the reaction After the end, the reaction solution was evaporated under reduced pressure to remove the solvent, the concentrate was filtered, and dried at 60° C. to obtain 37.5 g of 3,5-diiodo-2-hydroxybenzoyl chloride. ...
Embodiment 2
[0030] 3, the ratio of the amount of 3,5-diiodo-2-hydroxybenzoic acid and two (trichloromethyl) carbonate feed materials is 1: 0.34,3, the ratio of 5-diiodo-2-hydroxybenzoic acid and catalyst feed materials The amount ratio is 1:0.01, the catalyst is N,N-dimethylformamide, the organic solvent is toluene, and the mass ratio of the organic solvent to 3,5-diiodo-2-hydroxybenzoic acid is 10:1.
[0031] In a 1000mL three-neck flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 39.0g (100mmol) of 3,5-diiodo-2-hydroxybenzoic acid and 10.1g (34mmol) of bis(trichloromethyl)carbonate , toluene 449.8mL (389.9g) and N,N-dimethylformamide 0.1g (1mmol), the temperature was raised to 100°C and the reaction was stirred for 8h, and the reaction was tracked by thin-layer chromatography (the developing solvent was petroleum ether: ethyl acetate=5: 1 (volume ratio)), after the reaction finishes, the reaction solution is evaporated under reduced pressure to remove th...
Embodiment 3
[0033]3, the ratio of the amount of 3,5-diiodo-2-hydroxybenzoic acid and bis(trichloromethyl) carbonate feed material is 1:1,3,5-diiodo-2-hydroxybenzoic acid and the ratio of catalyst feed material The amount ratio is 1:0.01, the catalyst is N,N-dimethylacetamide, the organic solvent is toluene, and the mass ratio of the organic solvent to 3,5-diiodo-2-hydroxybenzoic acid is 5:1.
[0034] In a 500mL three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 39.0g (100mmol) of 3,5-diiodo-2-hydroxybenzoic acid and 29.7g (100mmol) of bis(trichloromethyl)carbonate , toluene 224.9mL (195.0g) and N,N-dimethylacetamide 0.1g (1mmol), warming up to 60°C and stirring for 6h, TLC tracking reaction (developing solvent is petroleum ether: ethyl acetate=5: 1 (volume ratio)), after the reaction finishes, the reaction solution is evaporated under reduced pressure to remove the solvent, the concentrate is filtered, and dried at 60°C to obtain 3,5-diiodo-2-h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com