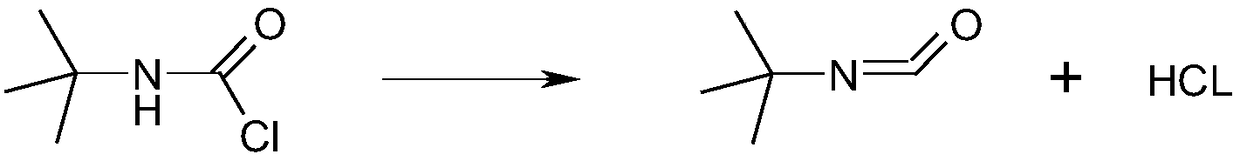
Synthesis method of tert-butyl isocyanate
A technology of tert-butyl isocyanate and butyl isocyanate, which is applied in the field of chemical synthesis, can solve problems such as difficulties in transportation and storage, hidden safety hazards, etc., and achieve the effects of reasonable process, safe production and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In the 1000ml four-necked flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, add the toluene of 2.4g triethylamine, 3.3 pyridine, 2.6g N-methylpyrrole and 249g to the four-necked flask, Start stirring and keep the stirring speed at 300 rpm, heat up to 90°C, add 135.5g of tert-butylcarbamoyl chloride, 27g of toluene, 0.3g of citric acid and 0.67g of p-toluenesulfonic acid into the constant pressure dropping funnel, at a temperature of 90°C Titrate for 3 hours, keep the reflux state at 90°C after the dropwise addition, continue to stir the reaction for 15 hours, and keep the stirring speed at 400 rpm. After the reaction is completed, send the mixed solution into a rotary evaporator. °C to 90 °C, washed with water three times, and dried in a vacuum oven at 80 °C for 9 hours to obtain 134.3 g of tert-butyl isocyanate with a yield of 92% and a content of 99.2%.
Embodiment 2
[0026] In a 1000ml four-necked flask equipped with mechanical stirring, a constant pressure dropping funnel, a reflux condenser and a thermometer, add 2.2g of triethylamine, 3.5g of pyridine, 2.8g of N-methylpyrrole and 2646g of dichlorobenzene, and start stirring And keep the stirring speed at 400 rev / min, heat up to 160°C, add 135.5g tert-butylcarbamoyl chloride, 294g dichlorobenzene, 0.54g citric acid and 0.98g p-toluenesulfonic acid into the constant pressure dropping funnel, at a temperature of 160°C Titrate for 2 hours, keep the reflux state at 160°C after the dropwise addition, continue to stir the reaction for 4 hours, and keep the stirring speed at 300 rpm. The temperature was 85°C, washed with water three times, and dried in a vacuum hollow drying oven at 90°C for 6 hours to obtain 135.8 g of tert-butyl isocyanate with a yield of 93% and a content of 99.5%.
Embodiment 3
[0028] In the 1000ml four-neck flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, add 2.9g of triethylamine and 3.2g of pyridine, 2.3g of N-methylpyrrole and 1622g of chlorobenzene, start Stir and keep the stirring speed at 500 rpm, heat up to 110°C, add 135.5g of tert-butylcarbamoyl chloride, 180g of chlorobenzene, 0.76g of citric acid and 1.14g of p-toluenesulfonic acid into the constant pressure dropping funnel and add dropwise at 110°C 2h. After the dropwise addition, keep the reflux state at 110°C and continue to stir the reaction for 11h. Keep the stirring speed at 300 rpm. After the reaction is over, send the mixed solution to a rotary evaporator for rotary evaporation. at 90°C, washed with water three times, and dried at 70°C for 9 hours in a vacuum hollow oven to obtain 137.2 g of tert-butyl isocyanate with a yield of 94% and a content of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com