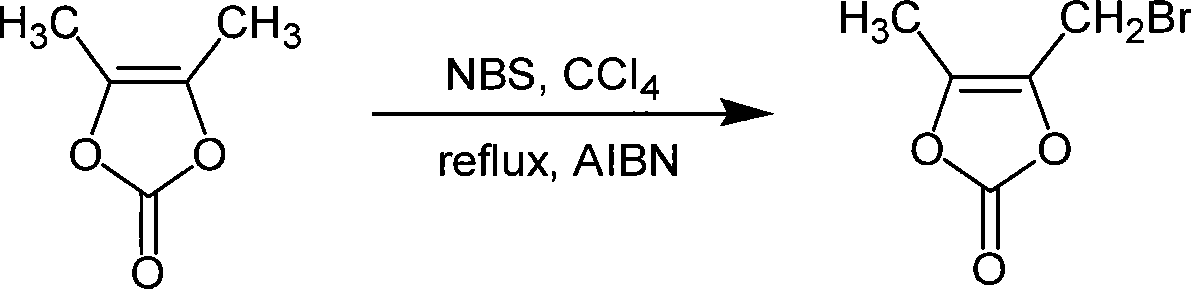
Chemosynthesis method of 4-bromomethyl-5-methyl-1,3-dioxy heterocyclic pentene-2-ketone
A technology of dioxole and bromomethyl, which is applied in the field of chemical synthesis of 4-bromomethyl-5-methyl-1,3-dioxol-2-one, can solve bromine Eliminate problems such as poor substitution selectivity, low reaction yield, and high toxicity of liquid bromine, and achieve the effects of less three wastes, simple operation, and safe and reliable production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The molar ratio of the feed material is DMDO:NBS is 1:1, the organic solvent is carbon tetrachloride; the initiator is azobisisobutyronitrile, and its dosage is about 5% of the DMDO mass; the reaction time is 15 minutes.
[0021] Add 500ml of carbon tetrachloride and 34.2g of DMDO to a 1L four-neck flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, then heat up and reflux. After reflux, add 1.71g (5%) initiator azobisisobutyronitrile (AIBN) and 53.4g N-bromosuccinimide (NBS), cool after reacting for 15 minutes, and the reaction liquid washes three times (3 * 500ml ), liquid separation, the organic layer was taken and dried with anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain a crude product. After rectification of the crude product, 48.6 g of the product 4-bromomethyl-5-methyl-1,3-dioxol-2-one was obtained, with a yield of 84% and a purity of 87.6%.
[0022] Structural characteri...
Embodiment 2
[0025] The molar ratio of the feed material is DMDO:NBS is 1:0.9, the organic solvent is carbon tetrachloride; the initiator is azobisisobutyronitrile, the dosage is about 8% of DMDO; the reaction time is 15 minutes.
[0026] Add 500ml of carbon tetrachloride and 34.2g of DMDO to a 1L four-neck flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, then heat up and reflux. After reflux, add 2.74g (8%) initiator azobisisobutyronitrile (AIBN) and 48.1g N-bromosuccinimide (NBS), cool after reacting for 15 minutes, and the reaction solution is washed three times with water (3 × 500ml ), liquid separation, the organic layer was taken and dried with anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain a crude product. After rectification of the crude product, 43.6 g of the product 4-bromomethyl-5-methyl-1,3-dioxol-2-one was obtained, with a yield of 75.3% and a purity of 87.1%.
Embodiment 3
[0028] The molar ratio of the feed material is DMDO:NBS is 1:1.1, the organic solvent is carbon tetrachloride; the initiator is azobisisobutyronitrile, and the dosage thereof is about 10% of DMDO; the reaction time is 15 minutes.
[0029] Add 500ml of carbon tetrachloride and 34.2g of DMDO to a 1L four-neck flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, and heat up to reflux. After reflux, add 3.42g (10%) initiator azobisisobutyronitrile (AIBN) and 58.7g N-bromosuccinimide (NBS), cool after reacting for 15 minutes, and the reaction solution is washed three times with water (3 * 500ml ), liquid separation, the organic layer was taken and dried with anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain a crude product. After rectification of the crude product, 42.2 g of the product 4-bromomethyl-5-methyl-1,3-dioxol-2-one was obtained, with a yield of 72.9% and a purity of 86.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com