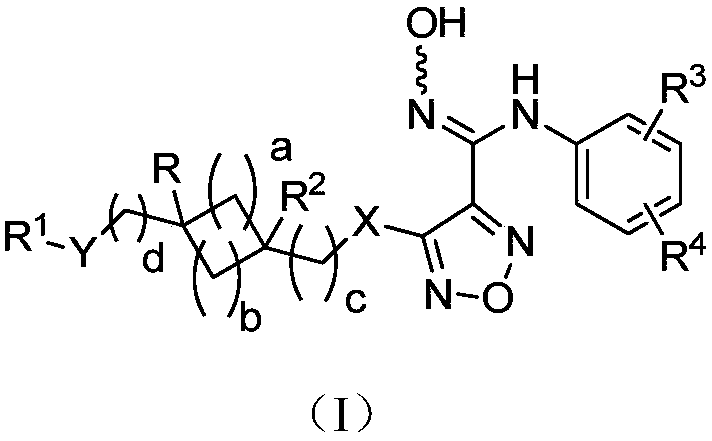
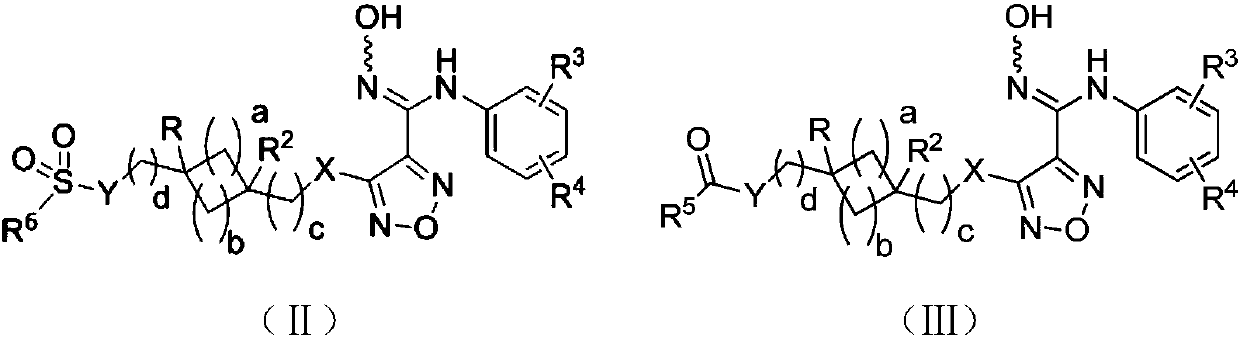
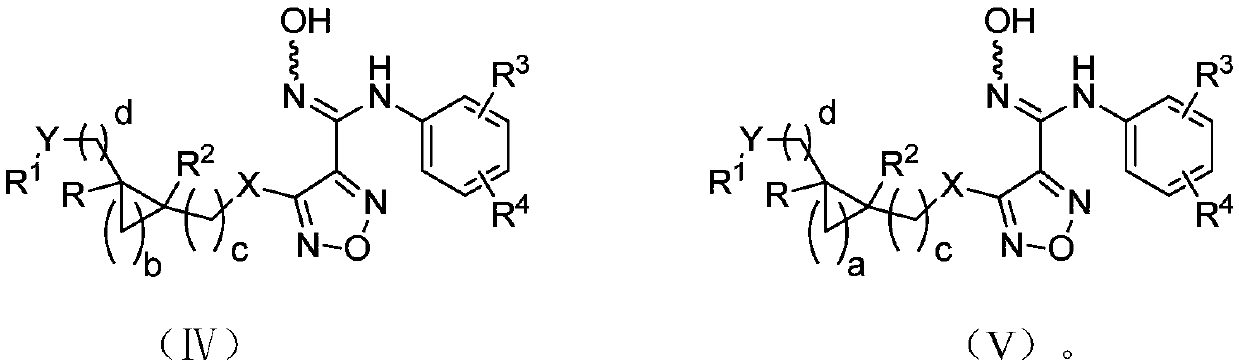
Indoleamine-2,3-dioxygenase inhibitor as well as preparation method and application thereof
A C3-C8, solvate technology, applied in the field of medicinal chemistry, can solve the problems of drug metabolism stability and toxicity, and achieve the effect of metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1, cis-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-(((-3-(sulfonamide)cyclobutyl)methyl)amino)-1,2 , Synthesis of 5-oxadiazole-3-amidine (compound 83)
[0047]
[0048] Compound 3-(dibenzylamino)cyclobutylcarboxylate methyl ester 83c was synthesized by literature method (WO 2015039348A1). 1. Synthesis of compound 3-oxocyclobutyl carboxylate methyl ester (83b)
[0049] Under the protection of nitrogen, the raw material compound 83a (4.0g, 35.1mmol) was dissolved in 40mL of dichloromethane and 5mL of methanol, and 2M TMSCHN was added dropwise under an ice-water bath. 2 (30 mL, 59.6 mmol). Stir in an ice-water bath for 2h. 1 mL of glacial acetic acid was added dropwise to quench the reaction, the solvent was spin-dried, and purified by silica gel column to obtain compound 3-oxocyclobutylcarboxylate methyl ester (83b) (4.4 g, 34.3 mmol), yield: 96.7%.
[0050] 2. Synthesis of compound 3-(dibenzylamino)cyclobutylcarboxylic acid methyl ester (83c)
[0051] The com...
Embodiment 2
[0071] Example 2, cis-4-(((-3-aminocyclobutyl)methyl)amino)-N-(3-bromo-4-fluorophenyl)-N'-hydroxyl-1,2,5- Synthesis of Oxadiazole-3-amidine (Compound 82)
[0072]
[0073] Compound cis-tert-butyl-3-(((4-(4-(3-bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2,4-oxadiazole -3-yl)-1,2,5-oxadiazol-3-yl)amino)methyl)cyclobutyl)formamide (83k) (40.0mg, 0.073mmol) was dissolved in 2.0mL methanol, added 2.5 N sodium hydroxide 1mL, stirred at room temperature for 1h. Ethyl acetate and water were added to extract. The organic layer was washed twice with brine, dried, and spin-dried to obtain 35 mg of a foamy solid. The foamy solid was added to 2.0 mL of TFA and 1.0 mL of dichloromethane, and stirred at room temperature for 1 h. The solvent was spin-dried, and extracted with water and dichloromethane. Take the water layer, adjust the pH to 8-9, add dichloromethane for extraction, dry the organic layer, and spin dry. The compound cis-4-(((-3-aminocyclobutyl)methyl)amino)-N-(3-brom...
Embodiment 3
[0076] Example 3, HC-2507-01: N-(3-bromo-4-fluorophenyl)-N'-hydroxyl-4-((((1s,3s)-3-hydroxycyclobutyl)methyl) Synthesis of Amino)-1,2,5-oxadiazole-3-amidine (Compound 90)
[0077]
[0078] 1. Synthesis of N-phenyl-3-oxocyclobutylformamide (90a)
[0079] Under nitrogen protection, 3-oxocyclobutylcarboxylic acid (83a) (5.0g, 43.8mmol), benzylamine (7.4g, 57.0mmol) and HATU (16.0g, 48.0mmol) were dissolved in 200mL DMF, added dropwise Diisopropylamine (11.7 g, 109.0 mmol). Stir at room temperature for 3h. Extracted with ethyl acetate and saturated water, the organic layer was washed twice with saturated brine, dried, spin-dried, and purified by silica gel column chromatography. N-phenyl-3-oxocyclobutylcarboxamide (90a) (4.5 g, 22.1 mmol) was obtained, yield: 50.5%. MS (ESI) m / e 204.3 (M+H)+.
[0080] 2, Synthesis of 3-((benzylamino)methyl)cyclobutanol (90b)
[0081] N-Phenyl-3-oxocyclobutylcarboxamide (90a) (4.5g, 22.1mmol) was dissolved in 150mL tetrahydrofuran, and lit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com