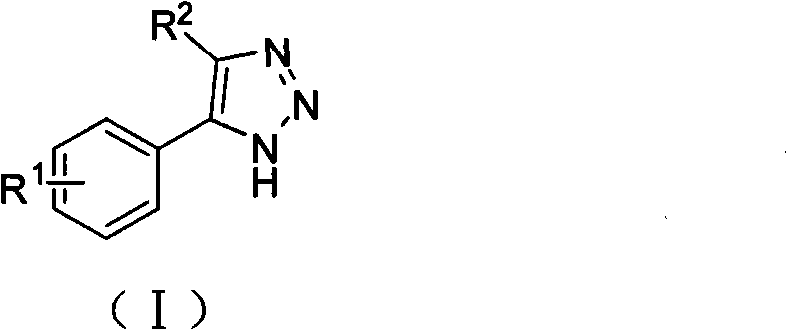
1, 2, 3-triazole compound and application thereof in preparing indoleamine 2, 3-dioxygenase inhibitor
A dioxygenase and compound technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of 4-phenyl-1H-1,2,3-triazole (compound 1)
[0037]
[0038] To a sealed tube filled with nitrogen, add trans-2,3-dibromo-3-phenylpropanoic acid (1 mmol) and 3 mL of DMF, followed by NaN 3 (4.0mmol), stirred at room temperature for 30min. Then add Xantphos (0.04mmol), Pd 2 (dba) 3 (0.01 mmol), sealed immediately, heated to 105° C. in an oil bath, and continued to heat for 30 h after the temperature was constant to synthesize 4-phenyl-1H-1,2,3-triazole with a yield of 71%. Among them: trans-2,3-dibromo-3-phenylpropionic acid and NaN 3 The molar ratio of trans-2,3-dibromo-3-phenylpropionic acid to Xantphos is 1:0.04; trans-2,3-dibromo-3-phenylpropionic acid to Pd 2 (dba) 3 The molar ratio of the reaction solvent DMF is 1:0.01; the mass ratio of the total volume of the reaction solvent DMF to trans-2,3-dibromo-3-phenylpropionic acid is 3 (mL): 1 (mmoL). The product is a white solid. 1 H NMR (500MHz, CDCl 3 +d 6 -DMSO): δ7.32-7.44 (3H, m),...
Embodiment 2
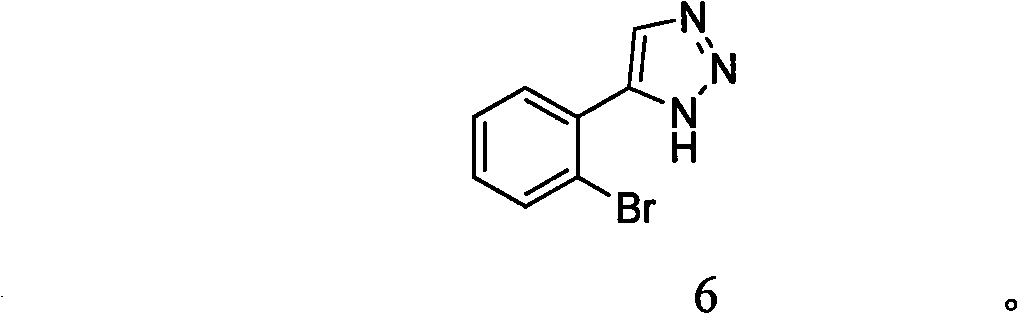
[0039] Example 2: Preparation of 4-(2-bromophenyl)-1H-1,2,3-triazole (compound 6)
[0040]
[0041] To a sealed tube filled with nitrogen, add trans-2,3-dibromo-3-(2-bromophenyl)propanoic acid (1 mmol) and 3 mL of DMF, followed by NaN 3 (4.0mmol), stirred at room temperature for 30min. Then add Xantphos (0.04mmol), Pd 2 (dba) 3 (0.01mmol), seal it immediately, heat the oil bath to 110°C, continue heating for 36h after the temperature is constant, and synthesize 4-(2-bromophenyl)-1H-1,2,3-triazole with a yield of 55%. Among them: trans-2,3-dibromo-3-(2-bromophenyl)propionic acid and NaN 3 The molar ratio of trans-2,3-dibromo-3-(2-bromophenyl)propionic acid and Xantphos is 1:0.04; trans-2,3-dibromo-3-( 2-Bromophenyl)propionic acid and Pd 2 (dba) 3 The molar ratio of the reaction solvent DMF is 1:0.01; the molar ratio of the total volume of the reaction solvent DMF to trans-2,3-dibromo-3-(2-bromophenyl)propionic acid is 3 (mL): 1 (mmoL). The product is a white solid. 1...
Embodiment 3
[0042] Example 3: Preparation of 4-(2-chlorophenyl)-1H-1,2,3-triazole (compound 8)
[0043]
[0044] To a sealed tube filled with nitrogen, add trans-2,3-dibromo-3-(2-chlorophenyl)propionic acid (1 mmol) and 3 mL of DMF, followed by NaN 3 (4.0mmol), stirred at room temperature for 30min. Then add Xantphos (0.04mmol), Pd 2 (dba) 3 (0.01mmol), seal it immediately, heat the oil bath to 110°C, continue heating for 36h after the temperature is constant, and synthesize 4-(2-chlorophenyl)-1H-1,2,3-triazole with a yield of 56%. Among them: trans-2,3-dibromo-3-(2-chlorophenyl)propionic acid and NaN 3 The molar ratio of trans-2,3-dibromo-3-(2-chlorophenyl)propionic acid and Xantphos is 1:0.04; trans-2,3-dibromo-3-( 2-Chlorophenyl)propionic acid and Pd 2 (dba) 3 The molar ratio of the reaction solvent DMF is 1:0.01; the molar ratio of the total volume of the reaction solvent DMF to trans-2,3-dibromo-3-(2-chlorophenyl)propionic acid is 3 (mL): 1 (mmoL). The product is a white so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com