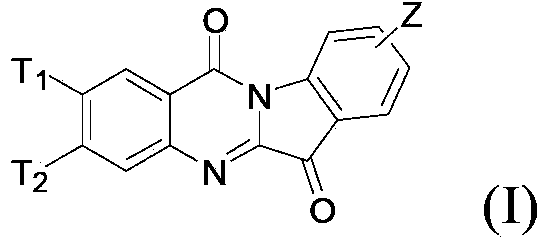
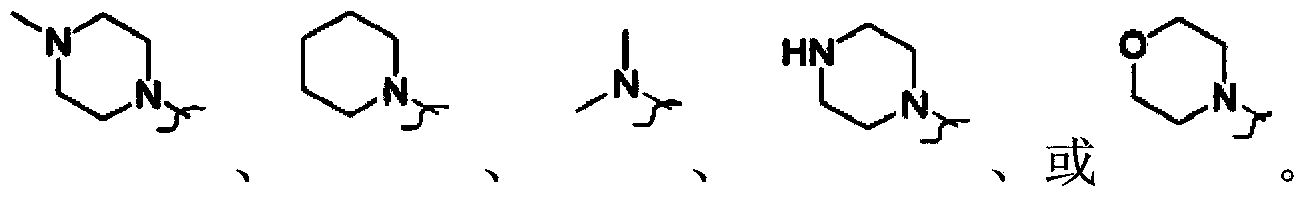N-alkyl tryptanthrin derivative, as well as preparation method and application thereof
A technology of alkyl and alkoxy groups, which is applied in the field of N-alkyl tryptanthrin derivatives and their preparation, and can solve problems such as insufficient development of tryptanthrin series compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The preparation method of the compound of formula I includes:
[0063]
[0064] (a) The compound of formula II undergoes an oxidation reaction to produce a compound of formula III;
[0065]
[0066] (b) The compound of formula III is reacted with fluoroisatin or isatin to produce compound of formula IV; and
[0067]
[0068] (c) The compound of formula IV undergoes substitution reaction to obtain the compound of formula I,
[0069] In each formula, R 1 , R 2 Independently selected from H, Br or I, and R 1 , R 2 Not at the same time as H;
[0070] T 1 , T 2 The definition of Z is as mentioned above.
[0071] Generally, in step a), the compound of formula II is oxidized in an organic solvent in the presence of an oxidizing agent at 20°C to 30°C for 2 to 3 hours to obtain a compound of formula III. The organic solvent is preferably selected from dichloromethane, tetrahydrofuran, methanol, ethanol, 1,4-dioxane, toluene, acetonitrile, isopropanol, n-propanol, acetic acid; the oxidizin...
Embodiment 1
[0086]
[0087] Compound 1 ((500mg, 2.2mmol) was suspended in 10ml of dry dichloromethane, and m-chloroperoxybenzoic acid (0.76g, 4.4mmol, 85%) was added in batches at zero degrees Celsius. Stir at room temperature for 2 Hours later. TLC(CH 2 Cl 2 / MeOH=50 / 1, Rf0.4) After the completion of the reaction was detected, the white solid formed by the reaction was filtered, and then washed with 10 ml of ethyl acetate three times to obtain compound 2 (420 mg, 79%).
[0088]
[0089] A mixture of compound 2 (3g, 12.4mmol), 5-fluoroisatin (2g, 12.4mmol) and triethylamine (3.6ml, 24.8mmol) was suspended in dry toluene (12ml) and heated at 110°C for 4 After hours, the solvent was distilled off under reduced pressure. The yellow solid was dissolved in 2 ml of dichloromethane, and then 2 ml of ethyl acetate was added. The yellow solid formed was filtered and used with 2 ml of ethyl acetate. After washing three times, the obtained yellow solid is compound 3 (1.9 g, 44%).
[0090] 1 H NMR(400MHz...
Embodiment 2
[0098]
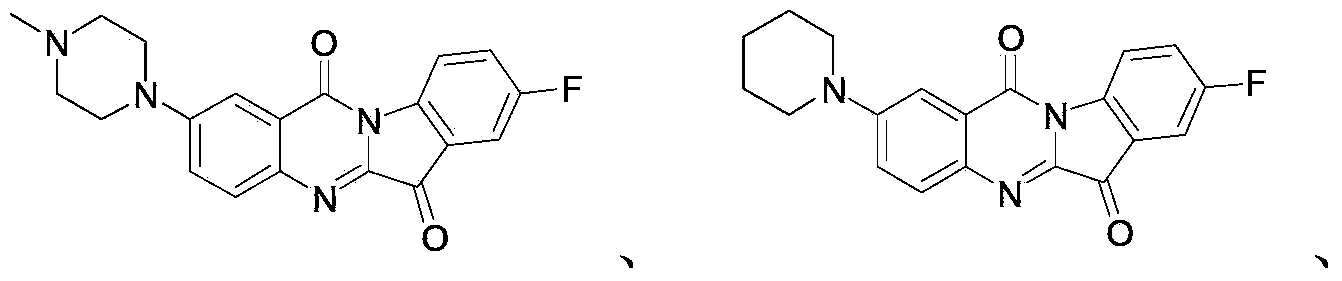
[0099] Under nitrogen protection, compound 3 (100mg, 0.29mmol), N-Methylpiperazine (N-methylpiperazine, 58mg, 0.58mmol), Pd(OAc) 2 (Palladium acetate, 20mg, 0.087mmol), BINAP ((±)-2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl, 84mg, 0.14mmol) and Cs 2 CO 3 (189mg, 0.58mmol) was suspended in anhydrous toluene (5ml) and heated at 110°C for 16 hours. TLC(CH 2 Cl 2 / MeOH=15 / 1, Rf0.4) indicates that the reaction is complete. After the solvent is evaporated under reduced pressure, 20 ml of dichloromethane is added to the resulting black solid, washed with water and brine, and dried. The black solid obtained after concentration is used Silica gel column (CH 2 Cl 2 / MeOH=15 / 1) separated to obtain compound 6 as a red solid.
[0100] 1 H NMR(400MHz, CDCl 3 )δ8.64(dd,J=8.8,4.1Hz,1H),7.90(d,J=9.1Hz,1H),7.76(d,J=2.9Hz,1H),7.57(dd,J=6.6,2.7 Hz,1H),7.47(td,J=8.7,2.7Hz,1H),7.39(dd,J=9.1,3.0Hz,1H),3.57-3.43(m,4H),2.69-2.57(m,4H) , 2.40(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com