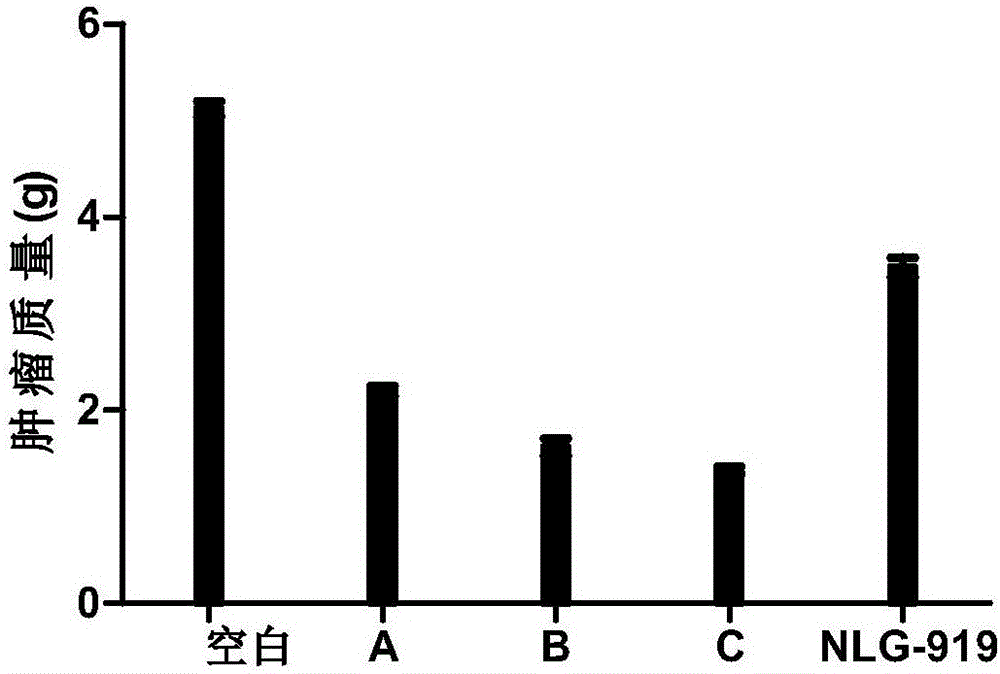
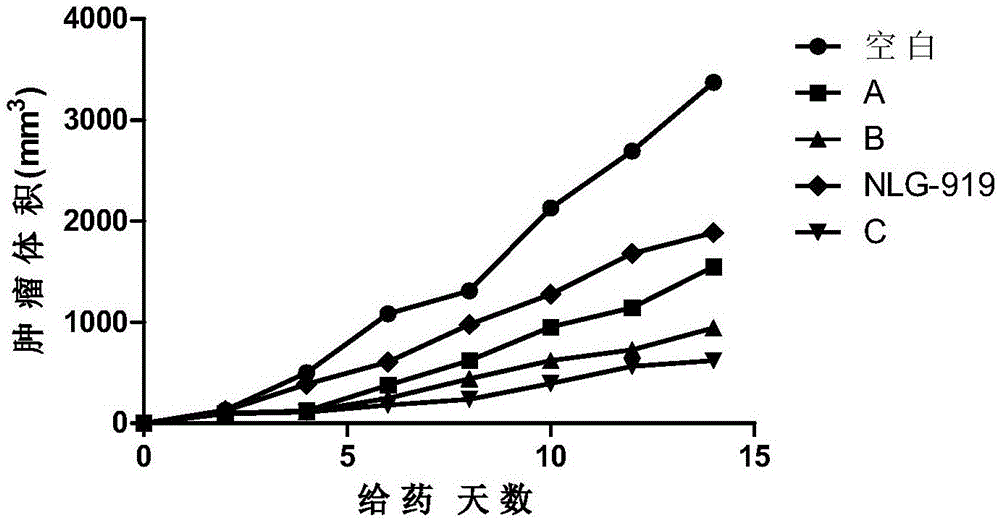
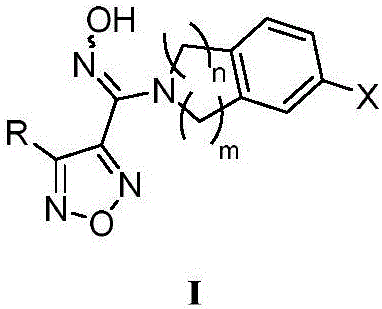
Nitrogen-containing benzoheterocyclic iodoleamine-2,3-dioxygenase 1 inhibitor and use thereof
A nitrogen atom, -NH2 technology, used in medical preparations containing active ingredients, organic active ingredients, nervous system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of 4-amino-3-(N'-hydroxyamidino)-1,2,5-oxadiazole(III)
[0057] Add sodium nitrite (20.7g, 300mmol) and 40mL water into a 500mL reaction flask, stir to dissolve, slowly add malononitrile (9.9g, 150mmol) hydrochloric acid (2N) solution dropwise at room temperature, stir overnight at room temperature, and cool to 0°C, add dropwise an aqueous solution of hydroxylamine hydrochloride (23g, 340mmol), stir for 30min, adjust the pH to 10 with 10N sodium hydroxide below 20°C, rise to 35°C for 2h, and then heat to reflux for 2h. Cool to room temperature, extract the reaction solution with 20 mL of ethyl acetate, distill off the solvent under reduced pressure to obtain a white solid, place the water layer overnight, precipitate a solid, filter with suction, wash with water, combine the filter cake with the white solid obtained by distillation under reduced pressure and dry to obtain a white powder like solid (14.9 g, 69.5%). 1 H NMR (300MHz, DMSO-d 6 )δ: 10.46(s,1H,-...
Embodiment 2
[0061] Preparation of Compound CPUL-I101
[0062] Preparation of 4-amino-3-(N-methyl-N'-hydroxyamidino)-1,2,5-oxadiazole (V-1)
[0063] Add IV (1.0g, 6.2mmol) and 40mL ethanol into a 100mL reaction flask, stir, and slowly add 40% methylamine ethanol solution (0.48g, 6.2mmol) and triethylamine (1.25g, 12.4 mmol), stirred at room temperature for 3 h, evaporated the solvent under reduced pressure, added ethyl acetate and stirred, filtered with suction, washed the filtrate once with water and saturated aqueous sodium chloride solution, dried the organic layer with anhydrous sodium sulfate, evaporated the solvent under reduced pressure to obtain yellow Oil (0.68g, 70.1%). HRMS m / z[M+H] + calculated for C 4 h 7 N 5 o 2 :157.0602,found:157.0600.
[0064] Preparation of 4-methylamino-3-(N'-hydroxyamidino)-1,2,5-oxadiazole (VI-1)
[0065] V-1 (0.68g, 4.3mmol) and 9mL of water were added to a 50mL reaction flask, and a 3mL aqueous solution of potassium hydroxide (0.73g, 13mmol) ...
Embodiment 3
[0071] Preparation of Compound CPUL-I102
[0072] Compound CPUL-I102 (38.9 mg, 15%) was synthesized in the same manner as compound CPUL-I101 except that ethylamine (0.95 mmol) was used instead of methylamine. m.p.187-188℃, 1 H NMR(300MHz,DMSO-d6)δ:10.99(s,1H,-OH),7.08-7.14(m,4H,-ArH),6.08-6.22(m,1H,-NH-),4.55(s, 2H,-CH 2 -),3.55(t,J=5.6Hz,2H,-CH 2 -),3.19-3.23(m,2H,-CH 2 -),2.88(t,J=5.6Hz,2H,-CH 2 -), 1.18(t, J=5.1Hz, 3H, -CH 3 )ppm.HRMS m / z[M+H]+calculated for C 14 h 17 N 5 o 2 :287.1380,found:287.1382.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com