Application of albiflorin as indoleamine 2,3-dioxygenase (IDO) inhibitor
A technology of paeonifloride glycosides and uses, applied in the field of paeonifloride glycosides as indoleamine 2,3-dioxygenase inhibitors, can solve side effects and other problems, achieve enhanced immune function, prevent and alleviate the formation and development of , the effect of preventing tumor cell escape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Effect of IDO inhibitor paeonifloride of the present invention on IDO overexpression and depression-like behavior induced by chronic stress rat model (CUMS)
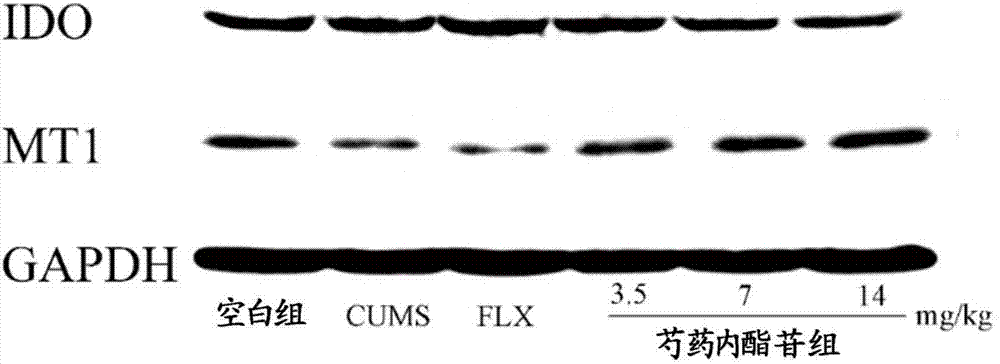
[0058] 1. In the hippocampal tissue of chronic unpredictable mild stress model (CUMS) rats, the secretion of IDO was significantly increased, and the melatonin receptor 1 (MT1) was significantly decreased. After 7 days of paeonifloride administration (7mg / day, 14mg / day), the IDO activity in the rat hippocampus was significantly inhibited (Pfigure 1 :
[0059] Effects of paeonifloride on indoleamine-2,3-dioxygenase (IDO) in rat hippocampus
[0060] Effect of melatonin receptor 1 (MT1) (Mean±SE)
[0061]
[0062] (# indicates an increase in P<0.05 compared with the model group, ## indicates an increase in P<0.01 compared with the model group, ** indicates a decrease in P<0.01 compared with the model group)
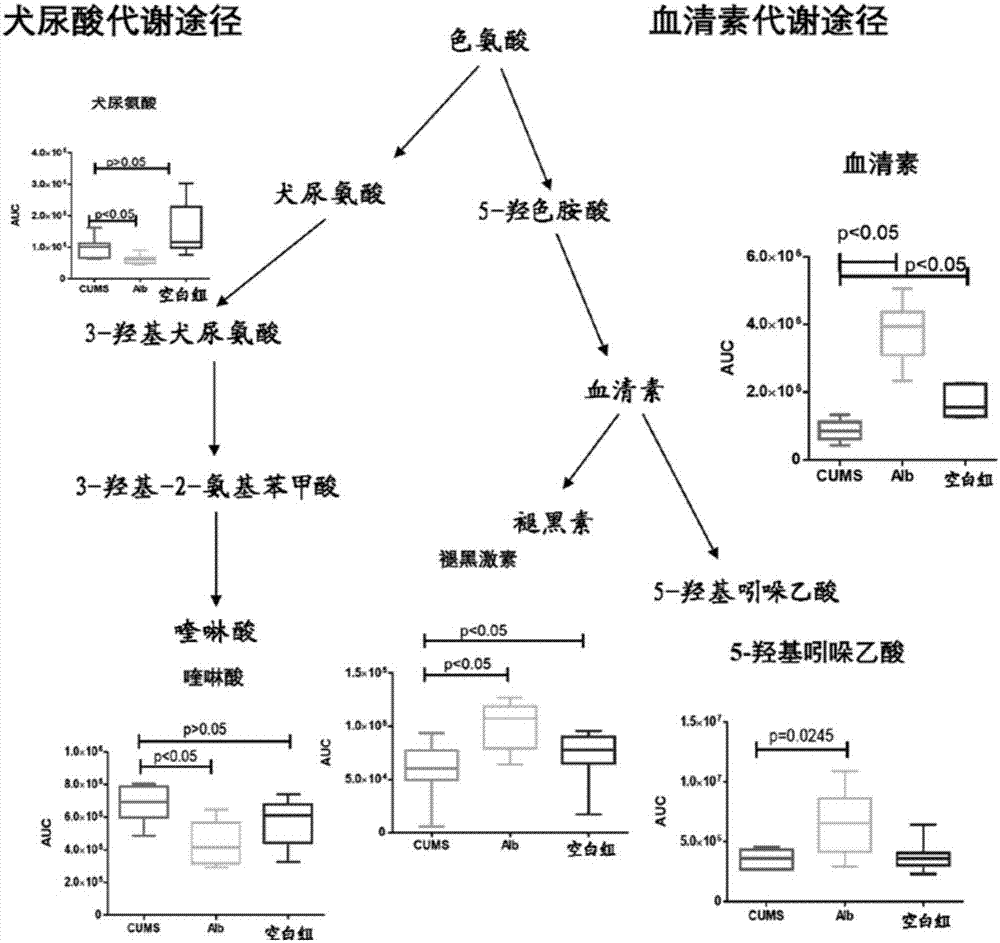
[0063] 2. The anabolic pathway of serotonin in the hippocampus of rats with chronic unpredictable mild s...
Embodiment 2
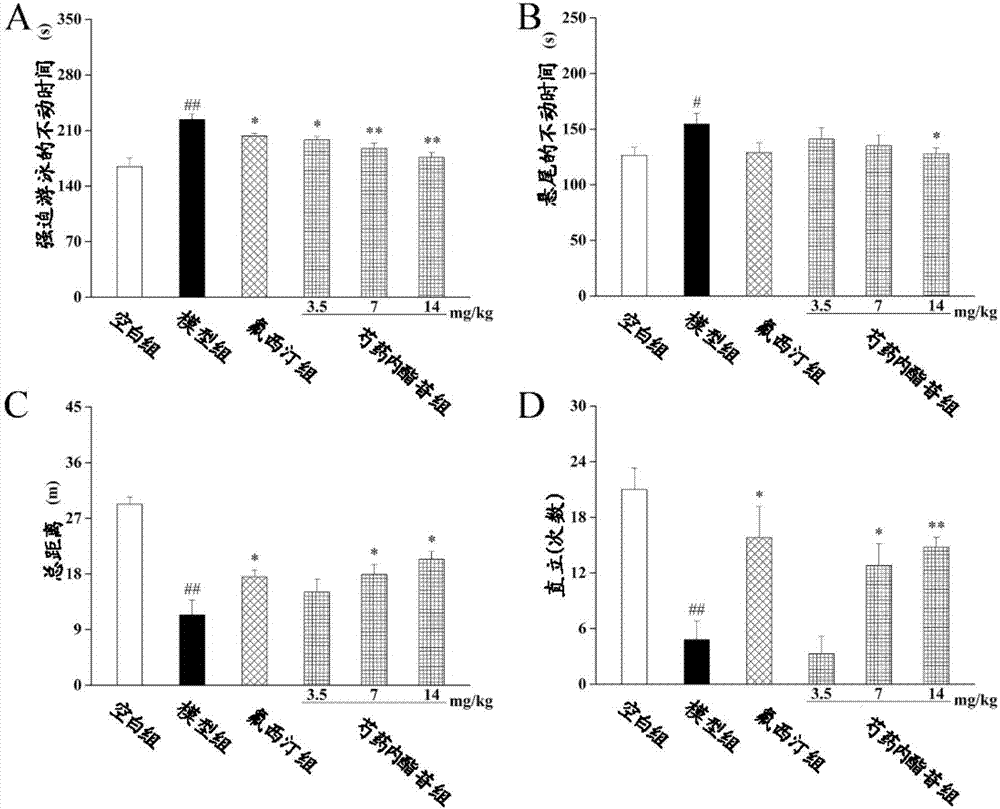
[0065] Effects of IDO inhibitor paeonifloride of the present invention on LPS-induced IDO activation, inflammatory depression-like behavior and cognitive dysfunction in mice
[0066] The mice were randomly divided into 6 groups (16 in each group): blank group, model group, positive drug fluoxetine hydrochloride group (10mg / kg), low-dose paeonifloride group (3.5mg / kg), middle-dose Group (7mg / kg), high-dose group (14mg / kg), continuous intragastric administration for 7 days.
[0067] 1. Experiment and testing content
[0068] 1. Behavioral experiments: forced swimming (FST), tail suspension (TST) and open field test (OFT)
[0069] After administration on the 7th day, intraperitoneal injection of LPS (0.83mg / kg) was used to establish the model, and 16 hours after the injection, the open field test (OFT) was performed; 24 hours after the injection, the forced swimming test (FST) and the tail suspension test (TST) were performed; after that , the eyeballs were picked to get blood,...
Embodiment 3
[0091] Embodiment 3. Molecular docking experiment of IDO1 inhibitor paeonifloride and IDO1 protein using MOE2016 software
[0092] 1. Selection of IDO1 crystal structure
[0093] At present, there are 20 crystal structures of IDO protein reported. After considering multiple factors such as biological source, resolution, and ligand small molecule structure, the crystal structure numbered 4PK5 was finally determined as the molecular docking structure of IDO1 protein.
[0094] 2. Small molecular structure, paeoniflorin (Albiflorin), the structure is as follows:
[0095] 3. Molecular docking experiments
[0096] The docking experiments and other related calculations were completed using the MOE2016 software package.
[0097] step:
[0098] Prepare the acceptor structure, correct errors in the structure, complete partial charge calculations and protonation procedures. Choose an appropriate subunit as the receptor and delete the rest.
[0099] Using the Flexible Alignment funct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com