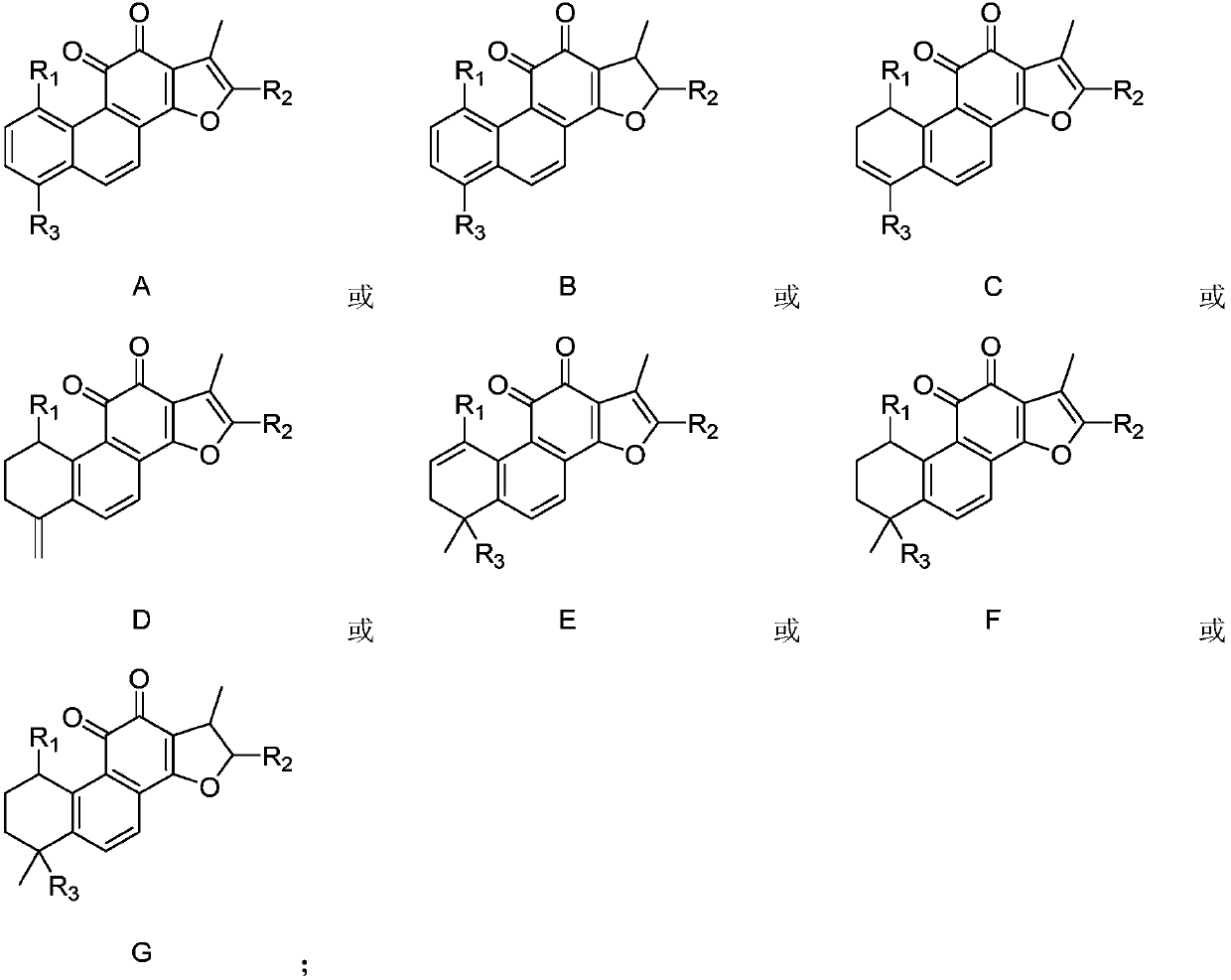
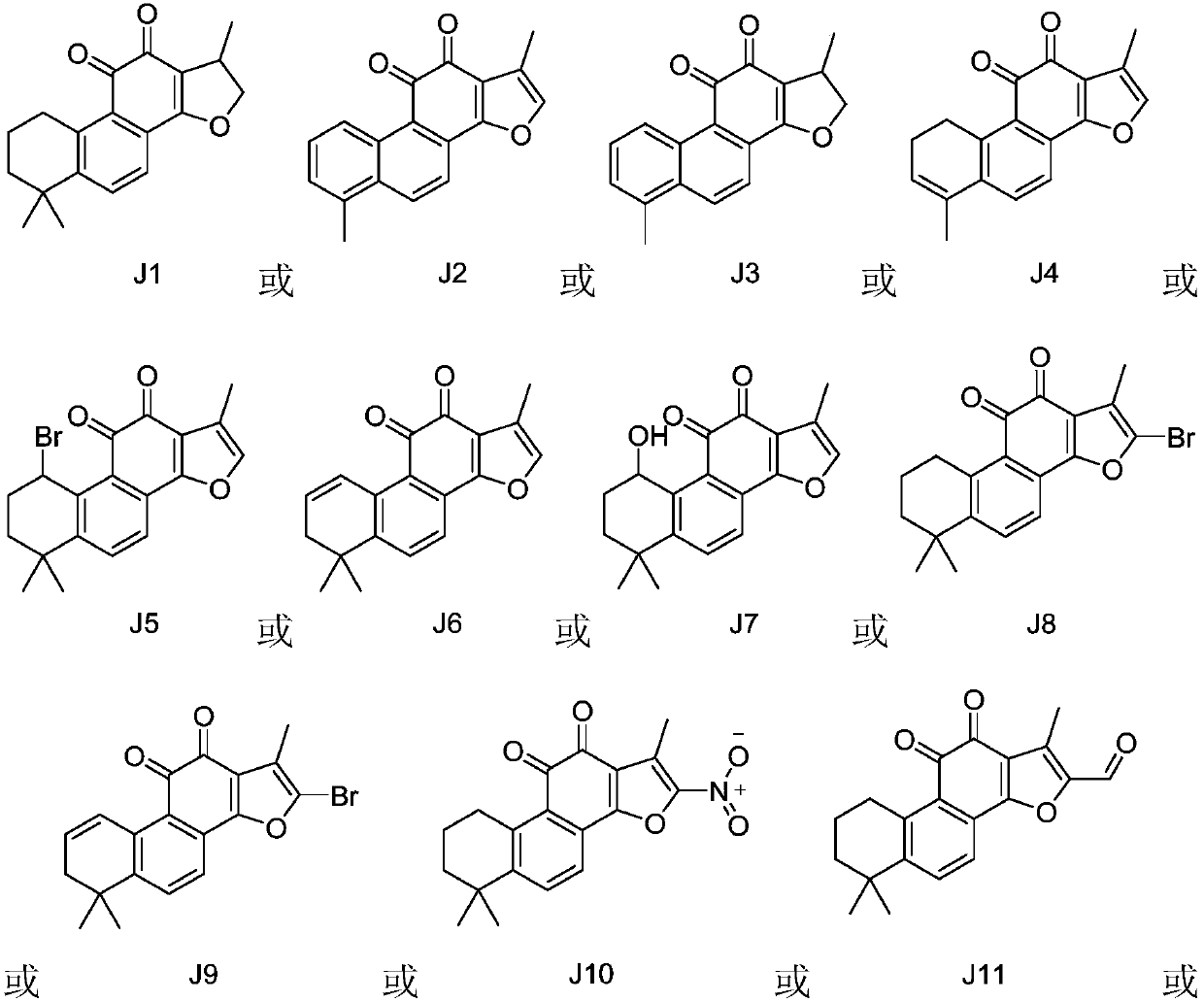
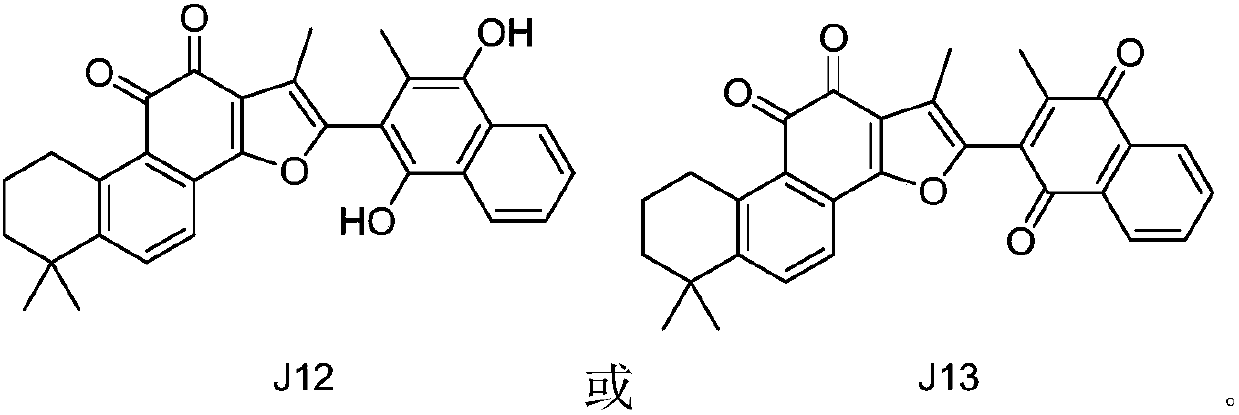
Indoleamine 2, 3-dioxygenase inhibitor containing tanshinone compound
A dioxygenase and compound technology, applied in steroids, medical preparations containing active ingredients, organic chemistry, etc., can solve the differences in physical properties, chemical properties and biological activities, and the structure-activity relationship needs further research and influence Physical properties, chemical properties and biological activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] In order to fully understand the technical content of the present invention, the technical solutions of the present invention will be further introduced and illustrated below in conjunction with specific embodiments.
[0023] The inhibitory activities of tanshinone compounds J1-J13 and compounds K1-K7 on indoleamine 2,3-dioxygenase were tested and evaluated respectively. The structures of compounds K1-K7 are shown below, respectively.
[0024]
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com