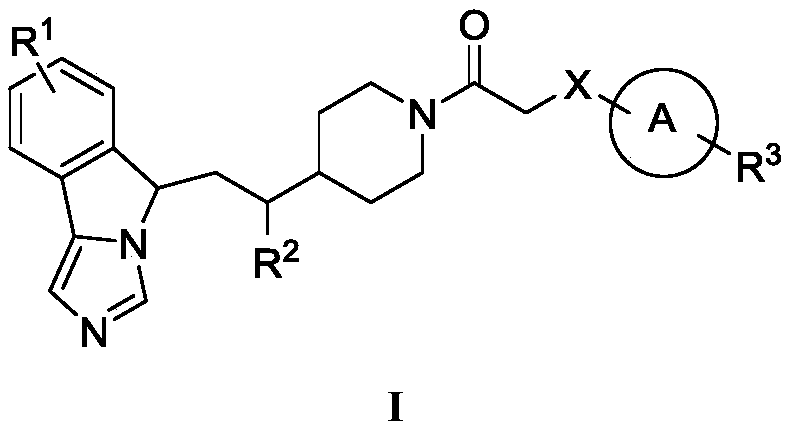
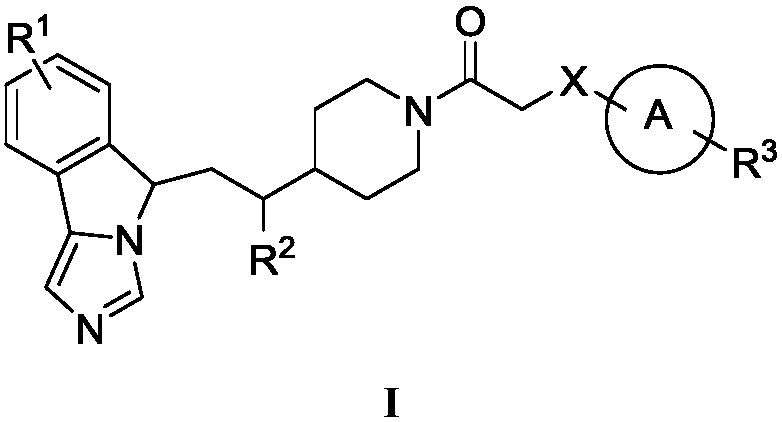
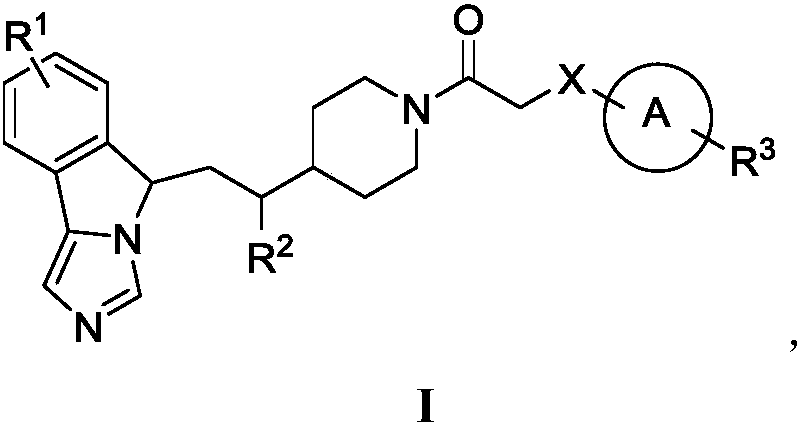
Fused imidazole compounds with indoleamine 2,3-dioxygenase inhibition activity
A compound, imidazolo technology, applied in the field of condensed imidazole compounds, can solve the problems of large toxic side effects and high dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Preparation of 4-iodo-1-trityl-1H-imidazole
[0144]
[0145] 4-Iodo-1H-imidazole (20 g, 103 mmoles) was dissolved in DMF (100 mL), then triethylamine (15.1 mL, 108 mmoles) and tritylmethyl chloride (27.8 g, 100 mmoles) were added. The reaction solution was stirred at room temperature for 48h, then poured into ice water (500mL), a large amount of solid was precipitated, filtered, and dried to obtain a white solid 4-iodo-1-trityl-1H-imidazole (40g, 91.7mmol, 88.9%).
[0146] LC-MS (m / z): 437 (M+1).
Embodiment 2
[0148] Preparation of 2-(1-trityl-1H-imidazol-4-yl)benzaldehyde
[0149]
[0150] 4-iodo-1-trityl-1H-imidazole (38.7g, 88.8mmol), (2-formylphenyl) boronic acid (20.0g, 133mmol) and K 3 PO 4 (56.4g, 266mmol) was dissolved in 1,4-dioxane (300mL) and water (60mL), nitrogen was bubbled into the reaction solution for 5min, and then Pd(PPh 3 ) 4 (5.12g, 4.44mmol), and continue to bubble nitrogen into the reaction solution for 5min. The reaction was carried out at 90° C. for 16 h under the protection of nitrogen. Cool down after the reaction, filter with diatomaceous earth, dilute the filtrate with water (100mL) and EA (300mL), let stand and separate the layers, extract the aqueous phase with EA (300mL×2; combine the organic phases, add water (100mL) and saturated saline (100mL×3) washing. The organic phase was concentrated under reduced pressure to obtain a residue, which was purified by column purification with PE / EA=3 / 1 to obtain light brown viscous oil 2-(1-trityl-1H-imida...
Embodiment 3
[0152] Preparation of (E)-4-(3-(2-(1-trityl-1H-imidazol-4-yl)phenyl)acryloyl)piperidine-1-carboxylic acid tert-butyl ester
[0153]
[0154] 2-(1-trityl-1H-imidazol-4-yl)benzaldehyde (4.0g, 9.66mmol) was dissolved in a mixed solution of anhydrous THF (20mL) and anhydrous EtOH (20mL), cooled to 0 °C, then EtONa (986 mg, 14.5 mmol), tert-butyl 4-acetylpiperidine-1-carboxylate (2.29 g, 10.1 mmol) were added. Then the reaction solution was stirred at room temperature overnight. After LCMS detected that the reaction was complete, it was diluted with ice water (30 mL), THF was distilled off under reduced pressure, filtered with suction, and the filter cake was drained as much as possible to obtain a white solid crude product (E)-4-(3 -(2-(1-Trityl-1H-imidazol-4-yl)phenyl)acryloyl)piperidine-1-carboxylic acid tert-butyl ester (6.0 g), used directly in the next step.
[0155] LC-MS (m / z): 624 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com