Application of quassia alkaloids serving as indoleamine-2,3-dioxygenase (IDO) inhibitor
A technology of dioxygenase and alkaloids, applied in the field of medical applications, can solve the problems such as no related reports on alkaloids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 In Vitro Activity Screening and Evaluation
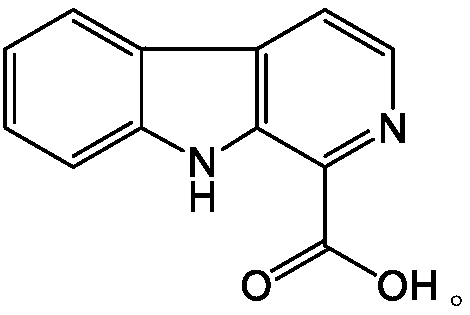
[0019] The IDO biological activity detection system was established, and the IDO recombined and purified in vitro was used as the research model, and the enzyme reaction kinetic monitoring method was used to measure the inhibitory activity of the alkaloids on IDO, and the Ki and IC were calculated. 50 value and further examine the type of inhibition for active compounds. The six kinds of bitterwood alkaloid compounds studied in the experiment have different structural skeletons, and the chemical structures are as follows:
[0020]
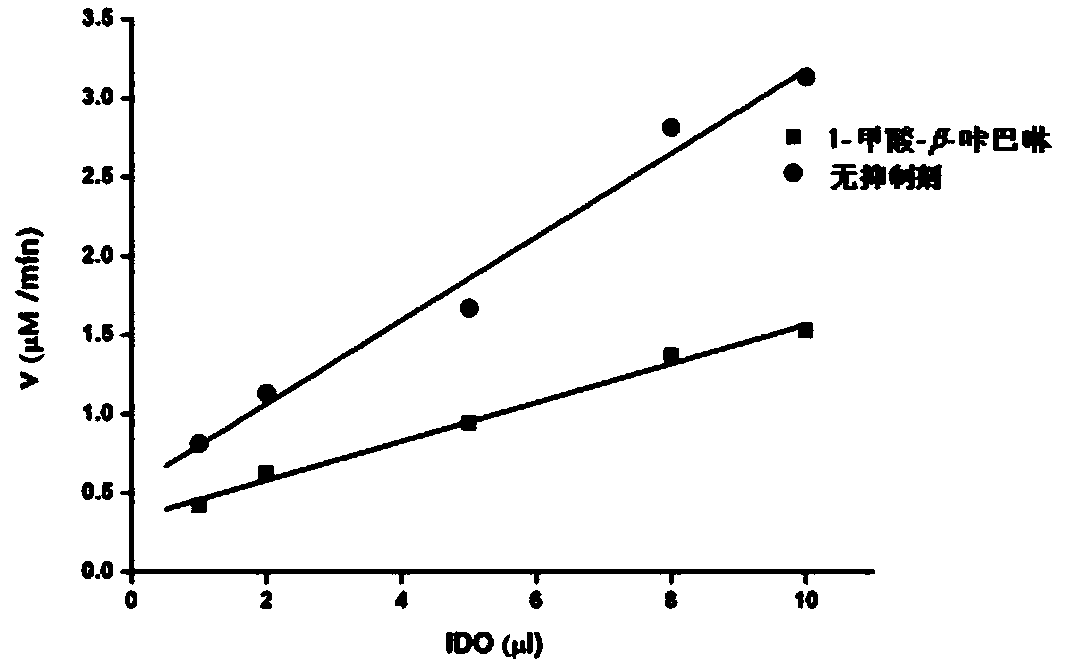
[0021] 1. Detection of IDO inhibitory activity
[0022] Reaction conditions: Mix 100mM potassium phosphate buffer (pH 6.5), 40mM vitamins, 200μg / mL catalase, 20μM methylene blue, 300mM substrate L-tryptophan and 100mM test sample, and incubate the mixture at 37°C for 5 minutes , then add IDO enzyme to the above mixture, react at 37°C for 30 minutes, add 200 μL of 30% (w / v) trichloro...
Embodiment 2
[0034]Example 2 In vivo activity evaluation
[0035] For the in vitro screening of active compounds, tumor mice were used as research models to investigate the effect of bitterwood alkaloids on tumor growth, and the ratio of kynurenine to tryptophan in serum was detected by HPLC to reflect the change of IDO activity, and the screening obtained high efficiency and low toxicity Novel IDO inhibitor drug candidates.
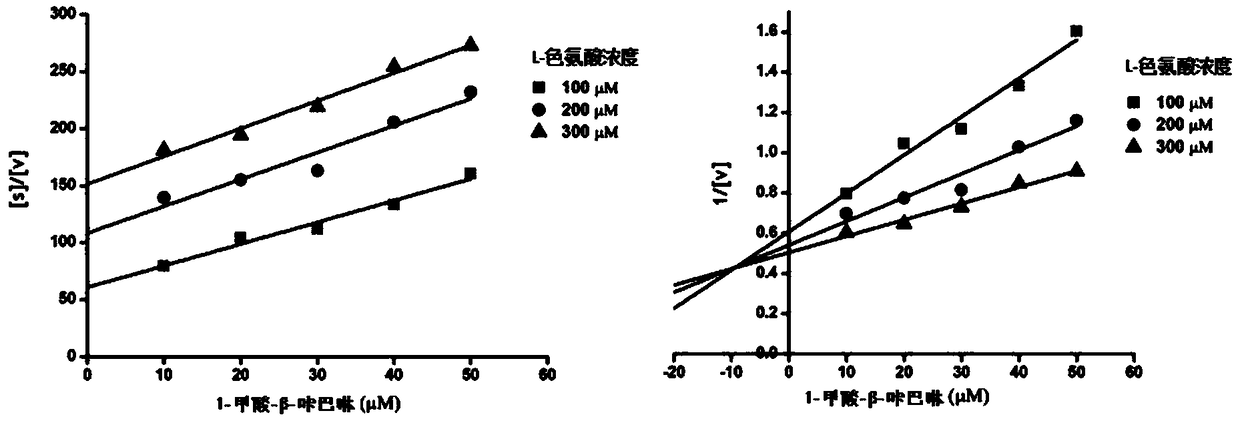
[0036] 1. Study on the inhibitory effect of 1-formic acid-β-carbaline on tumor growth
[0037] Take the human lung cancer A549 cells in the logarithmic growth phase, count the cells, put them into a 50mL centrifuge tube, resuspend them in 50% PBS buffer (pH 7.4) and 50% Matrigel, and adjust the cell concentration to 8×10 7 Cells / mL, pipette the cells to disperse them evenly. Aspirate the cell suspension and inject it into the anterior right mammary fat pad of healthy female Balb / C nude mice, inoculate 150 μL (1.2×10 7 cells / mL only). Select 30 tumor-bearing mice ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com