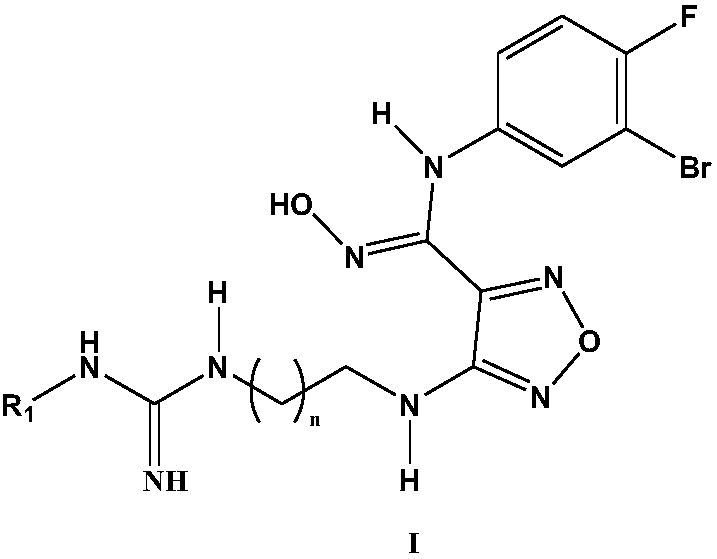
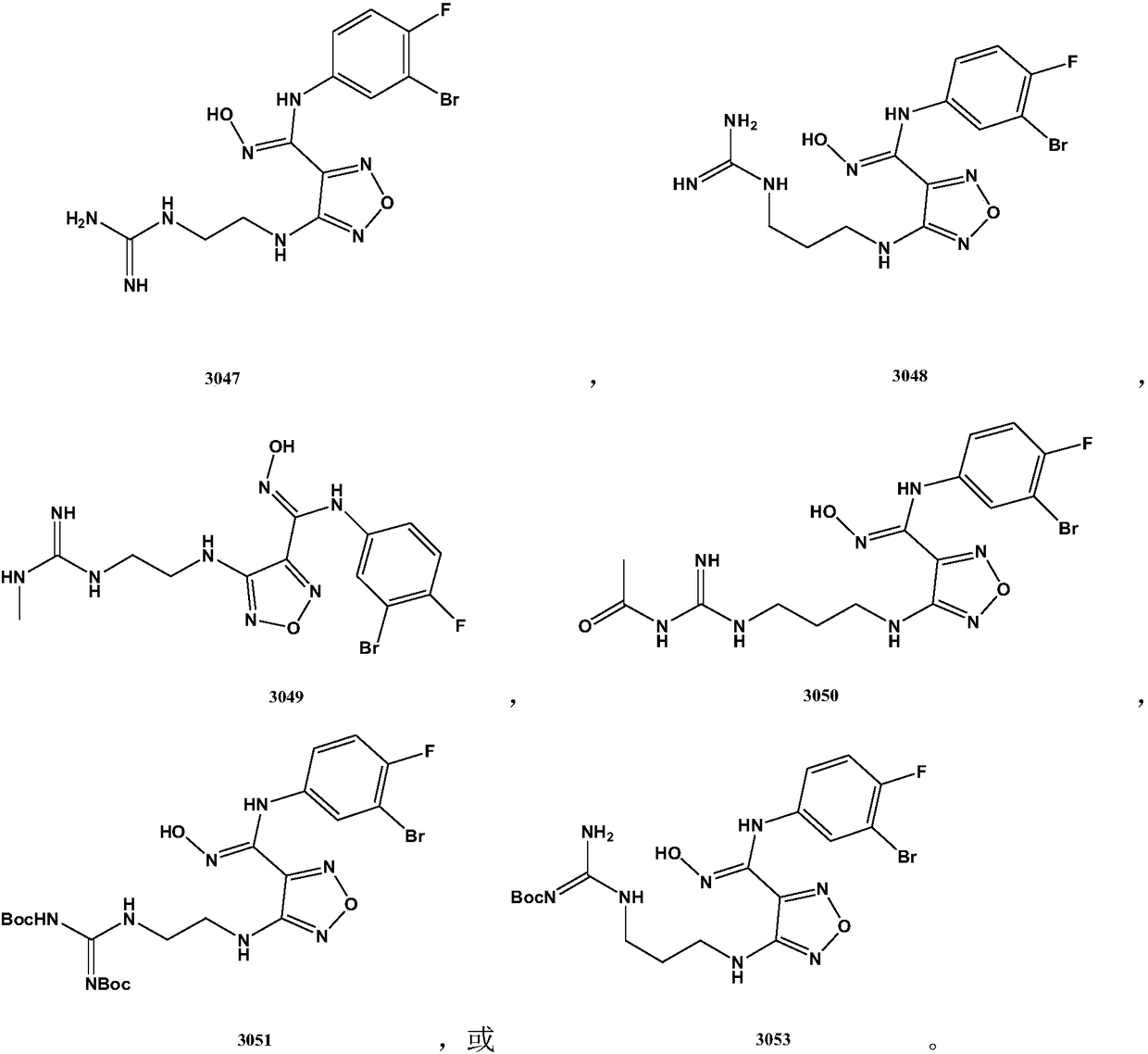
Carbamidine derivative
A technology of amino and compound, applied in the field of imide urea derivatives and its preparation, can solve the problems of unsatisfactory clinical curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13047
[0065] The synthesis of embodiment 1 3047
[0066] Response 1
[0067]
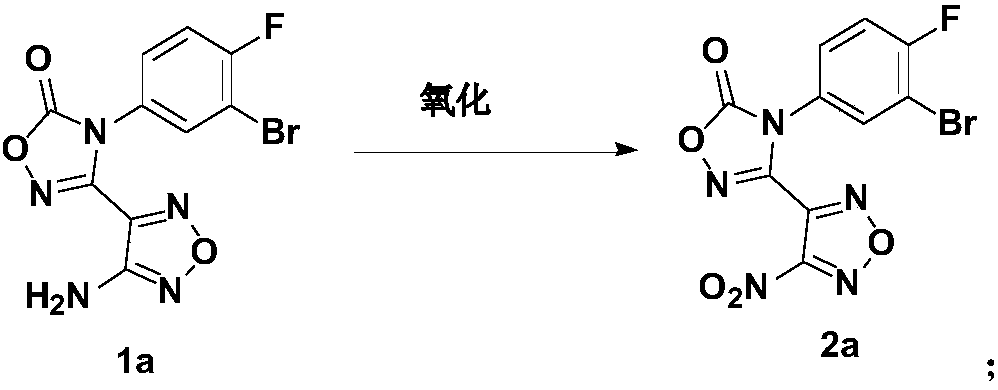
[0068] Compound 1a (682 mg, 2 mmol) was dissolved in trifluoroacetic acid (13 mL), and then 30% H 2 o 2 , reacted overnight at 50°C, the reaction solution changed from turbid to a clear yellow solution, after the reaction was completed, it was quenched with saturated sodium sulfite solution, and after the KI starch test paper showed colorless, it was extracted with ethyl acetate (50mL*2), and the organic phase was extracted with Dry over anhydrous sodium sulfate, concentrate and column chromatography (petroleum ether→petroleum ether: ethyl acetate = 1 ether: ethyl: 1) to obtain light yellow solid 2a (500 mg, yield 67%).
[0069] response 2
[0070]
[0071] Ethylenediamine 1 (30mg, 0.5mmol) was added to a solution of compound 2a (170mg, 0.5mmol) in tetrahydrofuran (10mL), then 1N NaOH (0.4mL) was added, the reaction solution was stirred at room temperature for 0.5h, and the reaction solution was ...
Embodiment 23048
[0078] The synthesis of embodiment 2 3048
[0079] Response 1
[0080]
[0081] Propylenediamine (35mg, 0.5mmol) was added to compound 1 (170mg, 0.5mmol, the synthesis method is detailed in the synthesis of compound 2a in XSD3-047Final report) in tetrahydrofuran (10mL) solution, the reaction solution was stirred at room temperature for 0.5h, and the reaction The liquid was directly prepared to obtain 130 mg of the target product.
[0082] response 2
[0083]
[0084] Compound 2 (130mg, 0.33mmol) and compound 1a (100mg, 0.33mmol) in methanol (10mL) were stirred overnight at room temperature, the reaction solution was concentrated and then column chromatographed (petroleum ether → petroleum ether: ethyl acetate = 1 ether: ethyl ether) : 1) The target product 3 (78 mg, 45%) was obtained.
[0085] Response 3
[0086]
[0087] Trifluoroacetic acid (0.6 mL) was added to a solution of compound 3 (31 mg, 0.05 mmol) in dichloromethane (3 mL), stirred overnight at room temp...
Embodiment 33049
[0088] The synthesis of embodiment 3 3049
[0089]Response 1
[0090]
[0091] Compound 1 (384mg, 1mmol, see the final report of XSD3-047 for the preparation method) and compound 1 (100mg, 1.1mmol) in tetrahydrofuran (10mL) were stirred at room temperature for 48h, and the reaction solution was directly prepared to obtain the target product 2 (89mg, Yield 20%).
[0092] response 2
[0093]
[0094] The compound (89mg, 0.2mmol) was dissolved in THF (10mL), then adjusted to pH=12 with 1N NaOH solution, and continued to stir for 20min. The reaction was monitored by LCMS. After the reaction was completed, the reaction solution was adjusted to neutral with 0.5N HCl. After the reaction solution was concentrated, the target product (13 mg, yield 15%) was prepared by pre-HPLC. target compound 1 H-NMR (400MHz, DMSO-d6): δ(ppm): 11.47(s, 0.6H), 8.92(s, 1H), 7.50-7.09(m, 5H), 6.70(s, 1H), 6.28(s , 1H), 2.72-2.50 (m, 4H), 2.50 (m, 3H). MS: m / z 416.2 [M+1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com